2013 Nobel Prize In Chemistry Goes To Trio That Took The Chemistry Lab Virtual
This year’s Nobel Prize in Chemistry goes to a trio of researchers that developed better computer models of chemical reactions. By helping scientists shrink the chemistry set into something that fits on a microchip, it’s easier than ever for chemists to simulate photosynthesis inside a plant, or the creation of a new drug.
On Wednesday, the Royal Swedish Academy of Sciences awarded the chemistry Nobel to Martin Karplus of the University of Strasbourg in France and Harvard University, Michael Levitt of Stanford University, and Arieh Warshel of the University of Southern California.
"This year's prize is about taking the chemical experiment to cyberspace," Royal Swedish Academy of Sciences secretary Staffan Normark said Wednesday, according to CBS.
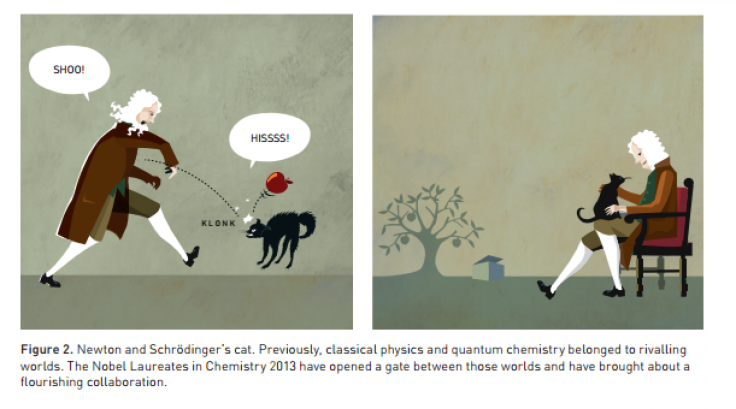
According to the Nobel committee, the trio’s computer models formed an important bridge between classical Newtonian physics and quantum chemistry. Newtonian-based computer programs were great for modeling the creation of large molecules, but not so adept at modeling the chemical reactions taking place -- the high-energy state of the molecules at play in a reaction doesn't really fall under the purview of classical physics. Conversely, simulations that use quantum physics offer a more realistic picture of how chemical reactions occur, but also require a lot of computing power.
“Classical and quantum chemistry were two fundamentally different, and in some respects rivaling, worlds,” the Nobel Committee for Chemistry said in a press release [PDF]. “But the Nobel Laureates in Chemistry 2013 have opened a gate between these two worlds. In their computer models, Newton and his apple collaborate with Schrodinger and his cat.”
Karplus and Warshel first started working together at Harvard in the 1970s. Warshel started working on new software based on a program he had made with Levitt during his doctoral training at the Weizmann Institute of Science in Rehovot, Israel. Eventually, he managed to create a computer model of what happens when retinal, a molecule embedded in the eye that changes shape when light hits the retina.
Levitt soon reunited with Warshel, and the pair was able to work on a program that could study the enzymes that drive biological reactions. They found a way to realistically simulate the complex reactions involving enzymes by weaving some very efficient code. One particular design feature of their program isn't requiring the computer to continuously monitor every atom of every component of every reaction. Merging several atoms together in a simulation for calculation purposes turns out to save on computing power.
The Nobel committee particularly liked that Karplus’, Levitt’s and Warshel’s work applies across all kinds of chemistry. One can use their methods to look at the behavior of enzymes as well as the chemical components of a solar cell or a cancer drug. A scientist can look at how some experimental unknown molecule will react to its environment -- whether that environment is inside a human body, or inside the battery of a car.
"The field of computational modeling has revolutionized how we design new medicines by allowing us to accurately predict the behavior of proteins," Dominic Tildesley, president-elect of Britain's Royal Society of Chemistry, told Reuters.
© Copyright IBTimes 2024. All rights reserved.











