Botox, The Anti-Wrinkle Drug, Has Been Approved By The FDA To Treat Crow’s Feet
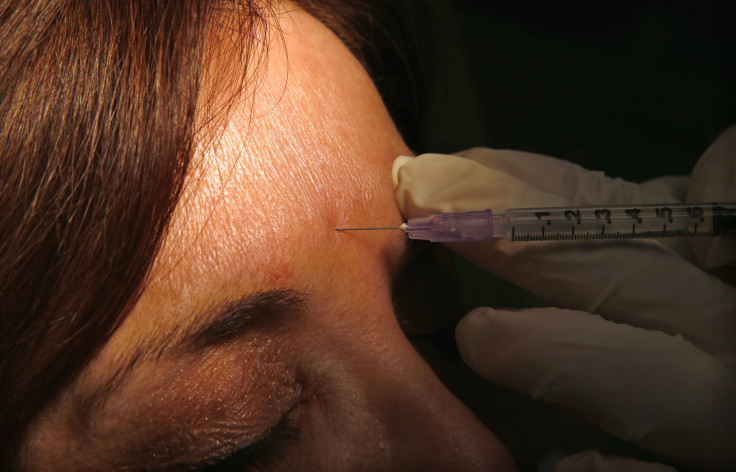
Botox, the anti-wrinkle drug, has been approved by the Food and Drug Administration, or FDA, to temporarily treat wrinkles around the eyes -- or crow’s feet as they are commonly known -- in adults.
The drug, which is marketed as Botox Cosmetic, was approved more than a decade ago for temporarily removing frown lines and wrinkles, besides treating medical conditions such as chronic migraine, underarm sweating and eyelid spasms.
“This additional indication will provide people with a new FDA approved treatment option for those seeking a smoother appearance by temporarily minimizing the appearance of crow’s feet at the sides of the eyes,” Susan Walker, director of the Division of Dermatology and Dental Products in the FDA’s Center for Drug Evaluation and Research, said in a press statement, on Wednesday.
But, doctors have been using Botox, or onabotulinumtoxinA, to smoothen crow’s feet without the FDA's approval for years now, Jennifer Walden, a Texas-based plastic surgeon, told CBSNews.
The product’s competitors -- Xeomin and Dysport -- have long been used to treat frown lines. But, Botox is the first to be approved to treat crow's feet, according to CBSNews.
Now, with FDA approving the drug, it gives “you an added benefit and safety of saying that I'm not doing anything illegal or potentially harmful," Marco Harmaty, a plastic and reconstructive surgeon at the Mount Sinai Medical Center in New York City, told CBSNews.
The anti-wrinkle effects of Botox Cosmetic, which temporarily paralyzes facial muscles to make wrinkles less prominent, lasts for about six months. However, there are side effects to using the drug, and the most common among them is eyelid edema, a condition where the eyelids become swollen from excessive fluid.
The drug is manufactured by Allergan Inc. (NYSE:AGN) based in Irvine, Calif., and FDA’s approval of the drug comes after two clinical efficiency and safety studies of the drug were undertaken.
On Wednesday, Allergan's stock, which is trading near its 52-week low, closed down 1.17 percent at $87.75 on the New York Stock Exchange.
© Copyright IBTimes 2024. All rights reserved.











