Chimerix Ebola Drug Brincidofovir Begins Testing In Liberia

In a break from traditional drug-approval procedures, health workers have gotten the OK to use experimental drugs from ongoing clinical trials to treat Ebola patients in West Africa. Global health authorities made the decision to allow pharmaceutical companies to test Ebola medicines on human patients in an attempt to quell the worst recorded outbreak of the often-fatal disease, which has already killed more than 8,000 people since March.
“With every possible treatment comes hope, and we are very excited that we may be able to help our patients beyond symptom management and routine supportive treatments like IV fluid therapy,” said Brett Adamson, of Doctors Without Borders (MSF), in a Thursday press release.
MSF has been administering the drug brincidofovir since Jan. 2 at its treatment center in Monrovia, Liberia. The drug is being developed by Chimerix Inc., which is based in Durham, North Carolina. The company’s researchers have been studying the compound as a treatment for a handful of medical issues, including smallpox, for which it has received a “fast track” designation from the U.S. Food and Drug Administration. This is the first time the drug will be tested on humans with Ebola.
As part of the West Africa trials, any patient who tests positive for the virus can volunteer to take the experimental medication. Unlike traditional drug trials where some patients receive a placebo version, all participants in the Ebola trials will be treated with the real drug.
“Randomizing patients to potentially receive a placebo was not considered ethical in the context of the current Ebola outbreak,” MSF said in a Thursday press release, noting that the results will be determined through comparison with patients treated at the clinic before brincidofovir was made available.
This is one of a rare few times when global health authorities have allowed doctors to use experimental drugs on patients. But experts say the current Ebola outbreak, which has killed more than 8,000 and affected more than 20,000 in Guinea, Sierra Leone and Liberia since March, merits an exception.
The World Health Organization noted in August that the West African outbreak is an “unprecedented” case, since local governments lacked the experience and infrastructure to deal with the virus, and there had been little research done on the disease in recent years.
“Before the outbreak, there was no commercial incentive to develop treatments or vaccines for filovirus diseases, and the lack of any such intervention has left public health authorities and clinicians in the affected countries with no specific prevention or treatment options, despite the fact that outbreaks have been occurring for nearly four decades,” the WHO said.
Since the Ebola outbreak began last year, Chimerix’s stock price has nearly doubled, even after experiencing a drop after the first American Ebola patient was treated with the company's drug but died soon after.
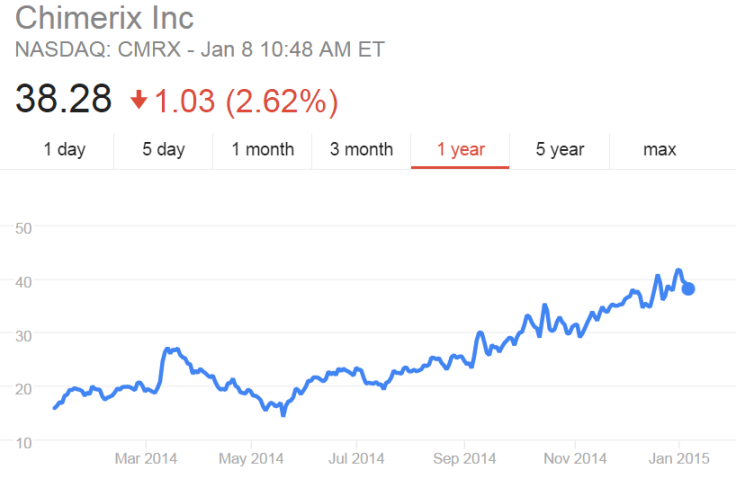
Chimerix is one of several pharmaceutical companies, including Tekmira Pharmaceuticals, Sarepta Therapeutics, NewLink Genetics and BioCryst Pharmaceuticals, that have seen their stocks rise in the past nine months as they scramble to find Ebola treatments or vaccines.
© Copyright IBTimes 2024. All rights reserved.












