Coffee Ring Effect Explained With Video (Photos and Video)
Ever wondered why a spilled drop of coffee dries out, forming a ring with a defined edge, as opposed to a drop of blood, which will dry as an evenly distributed blob? It took about a decade for scientists to explain this seemingly simple phenomenon, the "coffee ring effect."
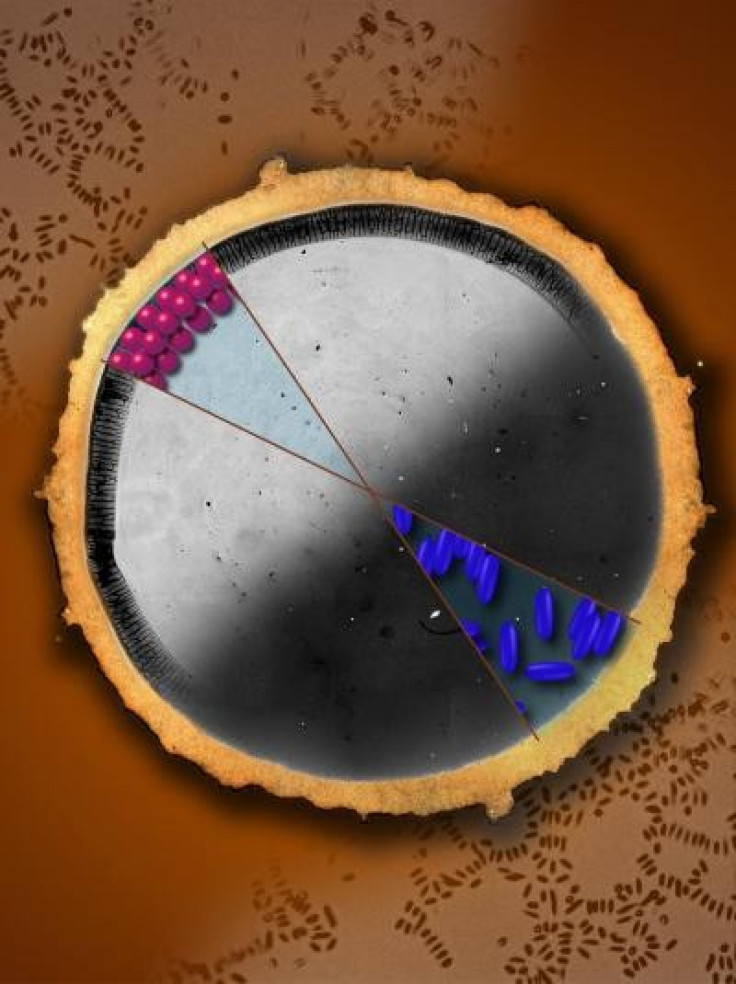
The edges of a coffee ring remain fixed as the liquid in the droplet evaporates. Since the volume steadily decreases, fluid flows outward from the middle of the droplet to its edges. This flow carries particles to the edges, and round particles at the edge will pack closely. By the time all of the liquid in the droplet evaporates, most of the particles will be at the edge, producing the coffee ring effect.
Researchers have determined that the shape of the particles in the liquid is an important factor in creating this pattern. This work, published in the August 18 issue of the journal Nature was performed by Arjun Yodh and colleagues at the University of Pennsylvania.
"We found that if you change the shape of the particles in the solution, the coffee ring effect goes away, and you end up with a uniform coating," said Peter Yunker, a graduate student in Yodh's lab.
The shape that liquid droplets take, and the way the shape changes as the droplets evaporate, is greatly influenced by surface tension at the air-liquid interface. This tension is a property of the interface, based on which the molecules in the liquid interact with one another versus the air. For example, liquids with a high surface tension, like water, may form a raised droplet, because the molecules are very attracted to one another and not so attracted to the air. In contrast, liquids with lower surface tension, like alcohols, are more likely to form flat spots instead of curved droplets.
The Yodh group found that elongated particles in a liquid behave differently than round ones because of the way they are affected by the surface tension of the air-liquid interface, the National Science Foundation says.
"If you make the particles elongated or ellipsoidal, they deform the air-water interface, which causes the particles to strongly attract one another. You can observe this effect in a bowl of cheerios. If there are only a few left, they clump together in the middle of the bowl, due to the surface tension of the milk," explained Yunker.
This clumping changes the way the particles distribute themselves within the droplet. Even if the clumped ellipsoidal particles reach the edge of the droplet, they do not pack as closely as round particles. The loosely packed clumps eventually spread to cover the entire surface, filling it so an even coating of particles is deposited when evaporation is complete.
"This work gives us a new idea about how to make a uniform coating, relatively simply. If you change the particle shape, you can change the way a particle is deposited. You can also make mixtures. In some cases, even just a small amount of ellipsoids can change the way the particles deposit when they dry," said Yodh.
The research results could eventually translate into new techniques or formulations for product coatings, or better inks and paints.
Coffee-ring Effect and its Suppression by Shape-dependent Capillary Interactions:
Abstract of the Video
Part I: Evaporating suspension of spheres is shown under bright field microscopy at a higher magnification. Individual spheres are seen as they are transported to the drop contact line, resulting in a ring-like deposit. This drop exhibits the coffee ring effect.
Part II: Evaporating suspension of ellipsoids is shown. Individual ellipsoids are seen adsorbing to the air-water interface where they form loosely-packed structures. The final deposition is relatively uniform. This drop does not exhibit the coffee ring effect.
Part III: A region near the contact line in an evaporating suspension of ellipsoids is shown. Individual ellipsoids are seen adsorbing to the air-water interface where they form loosely-packed structures. These structures prevent ellipsoids from reaching the contact line. This drop does not exhibit the coffee ring effect.
Part IV: A region near the contact line in an evaporating suspension of spheres is shown. Individual spheres are seen packing closely at the contact line, resulting in a ring-like deposit. This drop exhibits the coffee ring effect.
Part V: A region near the contact line in an evaporating suspension of ellipsoids with added surfactant is shown. The added surfactant decreases the surface tension. As a result, individual ellipsoids are seen packing closely at the contact line. Unlike suspensions of ellipsoids absent surfactant, this drop exhibits the coffee ring effect.
Video Credit: YouTube (Supplementary information of the paper "Suppression of the coffee-ring effect by shape-dependent capillary interactions").
© Copyright IBTimes 2024. All rights reserved.












