Deriving Fuel From Air: Converting Methane To Methanol May Become Simpler

Even as the continued use of fossil fuels (not that there are large-scale alternatives available everywhere, at least not just yet) wreaks havoc on the environment and the increasing amount of carbon — both carbon dioxide and methane — in the atmosphere exacerbates climate change, scientists are at work, trying to develop new energy sources.
One of the directions in which this research has gone is the attempt to convert methane into methanol. As a gas, methane (one atom of carbon bound to four hydrogen atoms) is abundantly found on the planet — as a component of natural gas, and also produced in a large number of human and animal activities.
In itself, methane is of no commercial use, and acts as a potent greenhouse gas. But if converted to methanol (one carbon and three hydrogen atoms bound to a hydroxide anion), it has many uses, including being a potential fuel. That conversion process is far from simple, though, typically requiring a large number of steps, which at a non-industrial scale, are neither economical nor efficient. And even the large-scale operations have to rely on very high temperatures that are expensive to generate.
Scientists from the Argonne National Laboratory, Illinois, have demonstrated a relatively cheaper and easier method to convert methane into methanol. In a recent paper published in the journal Nature, Argonne’s Sungsik Lee and his colleagues looked at “the potential of rhodium-based catalysts for this conversion under milder conditions.”
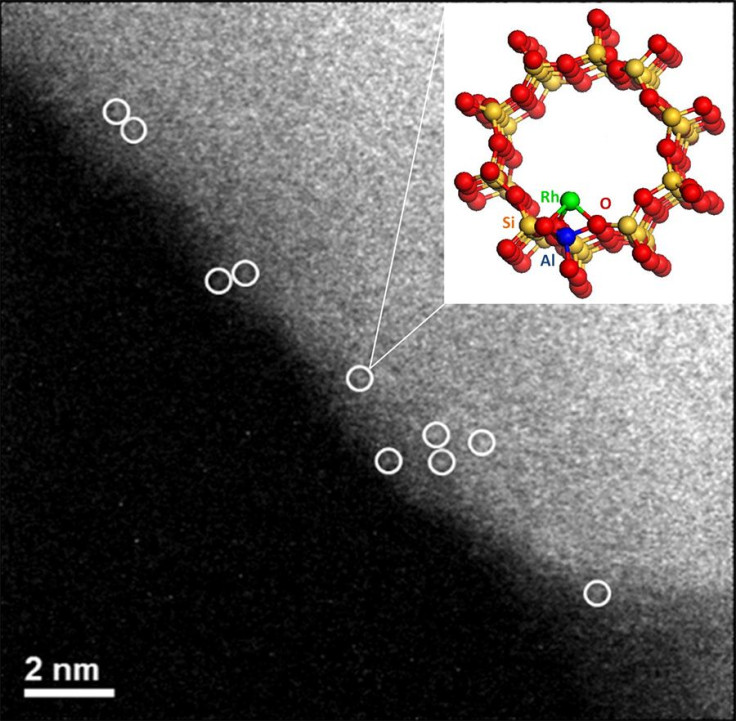
Lee, an X-ray scientist at Argonne, said in a statement Thursday: “Our work shows the potential of rhodium to enable this conversion under ‘mild conditions’ such as lower temperatures. Converting methane to methanol under mild conditions could have significant applications and present a breakthrough in catalysis.”
The rhodium catalysts, which were prepared using relatively simple methods, allowed for better conversion of methane to methanol and acetic acid (vinegar), when using oxygen and carbon monoxide under mild conditions. But the researchers were clear in knowing that more work is needed before their method can be widely used.
“While our work is still far from commercial application, it may inspire research directions for new methane-converting catalysts,” Lee said in the statement.
Methanol is an important ingredient in the production of a variety of chemicals, some of which are used to make consumer products like paints, plastics and plywood. Potentially, the gas can also fuel vehicles or be used to produce high grade hydrogen for fuel cells.
The paper, titled “Mild oxidation of methane to methanol or acetic acid on supported isolated rhodium catalysts,” also involved researchers from Tufts University, Massachusetts, and the Oak Ridge National Laboratory, Tennessee.
© Copyright IBTimes 2024. All rights reserved.











