Theranos CEO Elizabeth Holmes Banned From Operating Labs For 2 Years
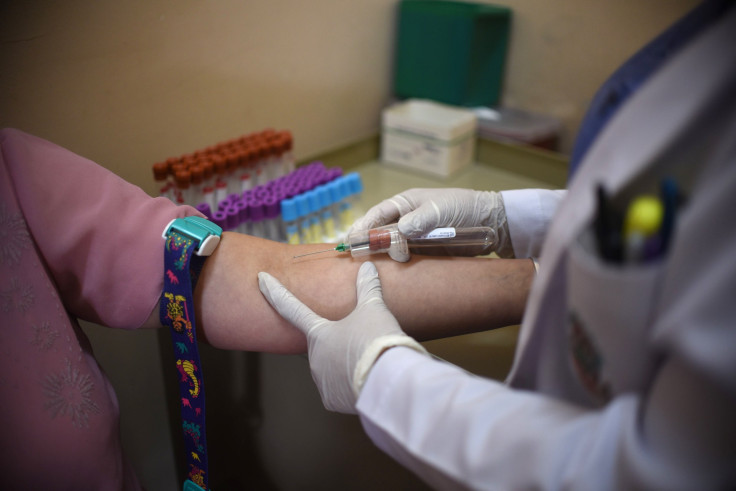
Federal health regulators have banned Theranos founder and CEO Elizabeth Holmes from operating a blood-testing laboratory for two years, revoked the company’s California lab’s regulatory approval and imposed a financial penalty on the company, among other measures, Theranos announced late Thursday.
Founded in 2003, Theranos is a health technology startup that became known for its blood-testing device called Edison. It was a game changer in the industry because according to the company, it uses only a few drops of blood to perform multiple tests, instead of the vials-full most people part with when they go for a blood test. That same device and its testing method became the center of a controversy following a Wall Street Journal report last year.
The ban imposed by the Centers for Medicare & Medicaid Services on the company and its founder relate to an investigation that started because of the controversy. In its statement Thursday, Theranos said sanctions by the CMS arose “from the 2015 survey of its Newark, California laboratory.”
The company also has been barred permanently from receiving “Medicare and Medicaid payments for all laboratory services” as part of the sanctions.
Revocation of the lab’s license will take effect in 60 days but “the company will not conduct any patient testing in the Newark lab until further notice. During this period, the company will continue to work with CMS to resolve and remediate outstanding issues in the Newark lab, and will continue to provide services to its customers through its Arizona lab,” Theranos said.

Holmes said in the statement: “While we are disappointed by CMS’ decision, we take these matters very seriously and are committed to fully resolving all outstanding issues with CMS and to demonstrating our dedication to the highest standards of quality and compliance.”
She added that the company would shut down the Newark facility and rebuild it from the ground up.
The company, valued at about $9 billion in 2014, was reduced to be worth only about $800 million by Forbes last month. Theranos also lost its big retail partner, Walgreens, recently as fallout of the controversy around its Edison machine. Walgreens was setting up Theranos testing centers in its pharmacy outlets, and had plans to roll them out nationwide.
Theranos is also under investigation from the U.S. Securities and Exchange Commission, the company confirmed in April.
© Copyright IBTimes 2024. All rights reserved.











