Alzheimer's Disease Trigger Mimics Mad Cow Infectious Agent: Study
Researchers using mice to explore the initial triggers of Alzheimer's disease believe they've fingered a prime suspect -- a highly toxic protein that converts other proteins into more toxic forms in a behavior that mimics mad cow disease.
These converted proteins then work in concert with another protein called tau to kill nerve cells according to a paper published in Nature on Wednesday. Nerve cell death is thought to be a primary cause of memory loss and other Alzheimer's symptoms.
The research was conducted by scientists from the University of Virginia and German biotechnology company Probiodrug.
The bad protein seed is called pyroglutamylated beta-amyloid, or pyroglu beta-amyloid for short. When researchers added it to cultured mouse nerve cells, the protein converted other beta-amyloids into a hyper-toxic form, and the converted proteins then turned and converted even more amyloids.
Two of the major characteristics of a brain damaged by Alzheimer's disease are aggregations of amyloid proteins and tau into plaques and tangles, respectively. But those plaques and tangles end-stage products, according to George Bloom, a University of Virginia biologist and senior author on the Nature paper.
In order to investigate the early causes of Alzheimer's Bloom and his team were observing the behavior of amyloids and tau proteins not as plaques or tangles, but as small aggregates called oligomers.
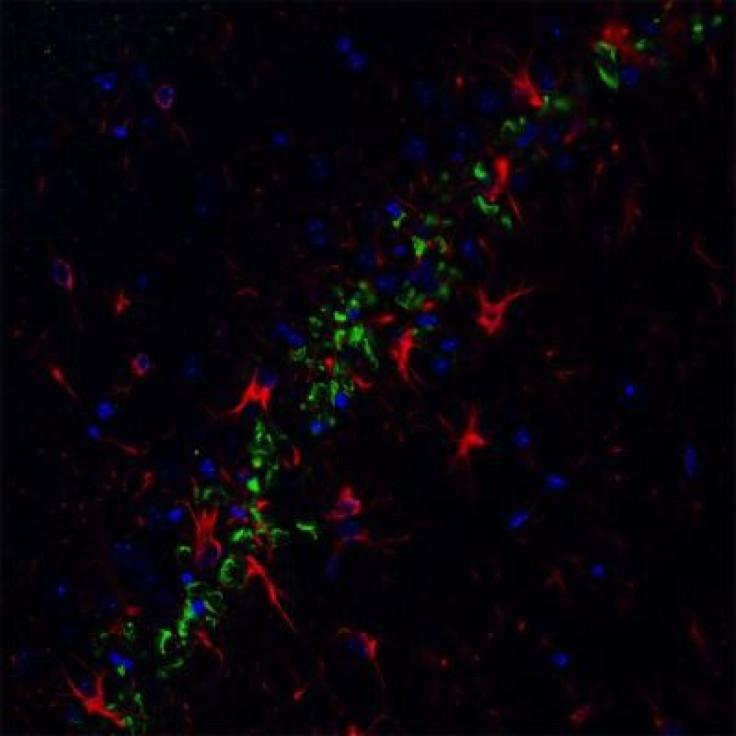
The scientists also looked at sections from the brains of mice that were bred to overproduce pyroglu beta-amyloid. Though the mice didn't have as much of the protein as a human Alzheimer's patient, there was a visible loss of nerve cells in the brains of the experimental mice compared to normal mice.
By the time the mice are three months old you see a massive loss of neurons in the hippocampus -- the center of memory in the brain, Bloom says.
However, another group of mice that were engineered to overproduce pyroglu beta-amyloid but also lacked the ability to make tau protein did not suffer nerve cell loss. Without the bullet of tau, the trigger of pyroblu beta-amyloid is rendered relatively harmless.
The next step, Bloom says, is to figure out how pyroglu beta-amyloid and tau are working together to damage brain cells.
Pyroglu beta-amyloid's ability to force other proteins to conform to a toxic template is similar to the strategy of prions, the oddly shaped proteins that cause mad cow disease in animals and Creutzfeldt-Jakob disease in humans.
The fact that it's a prion-like spread of disease means it's harder to stop because it spreads so quickly within the brain, says Bloom.
The prion-like behavior implicated in Alzheimer's disease also suggests that it may be transmissible like mad cow disease.
In October 2011, a group from the University of Texas Health Science Center at Houston reported in the journal Molecular Psychiatry that they could induce Alzheimer's disease in mice by injecting brain tissue from a human Alzheimer's patient.
The mice then developed amyloid plaques and other Alzheimer's-like brain alterations, while mice injected with normal brain tissue did not.
Our findings open the possibility that some of the sporadic Alzheimer's cases may arise from an infectious process, senior author Claudia Soto said in a statement in October.
© Copyright IBTimes 2024. All rights reserved.











