Coronavirus Treatment: FDA Issues Emergency Authorization Of Trump-Backed Anti-Malaria Drugs
KEY POINTS
- FDA issued an emergency use authorization of two anti-malaria drugs for the treatment of the deadly virus
- The two drugs, hydroxychloroquine and chloroquine, were championed by President Donald Trump
- However, experts feel there is very little evidence to prove the drugs are effective in treating COVID-19
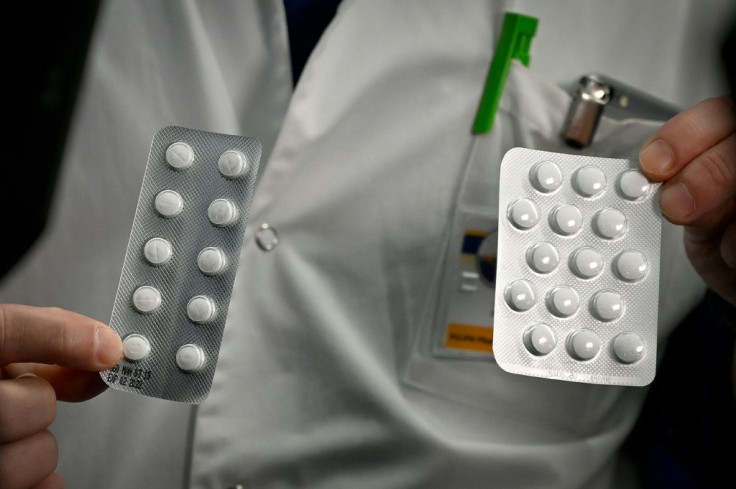
With the United States leading the world in terms of confirmed coronavirus cases, the Food and Drug Administration (FDA) issued an emergency use authorization of two anti-malaria drugs for the treatment of the deadly virus.
The two drugs, hydroxychloroquine and chloroquine, were championed by President Donald Trump. At a White House press briefing last week the president called the drugs “game-changer” that have shown “very, very encouraging results. ″
In a statement Sunday, the U.S Department of Health and Human Services (HHS) said FDA “allowed for the drugs to be donated to the Strategic National Stockpile to be distributed and prescribed by doctors to hospitalized teen and adult patients with COVID-19, as appropriate, when a clinical trial is not available or feasible.” HHS added that pharmaceutical companies Bayer and Sandoz had donated the drugs to the stockpile.
“Hydroxychloroquine sulfate and chloroquine phosphate are oral prescription drugs approved to treat malaria and other diseases. Although there are no currently approved treatments for COVID-19, both drugs have shown activity in laboratory studies against coronaviruses, including SARS-CoV-2 (the virus that causes COVID-19),” the statement added.
Stressing that Trump is “taking every possible step” to protect the citizens, HHS Secretary Alex Azar said, “Scientists in America and around the world have identified multiple potential therapeutics for COVID-19, including chloroquine and hydroxychloroquine. The President’s bold leadership and the hard work of FDA and HHS’s Assistant Secretary for Preparedness and Response have succeeded in securing this large donation of medicine. We’ll continue working around the clock to get American patients access to therapeutics that may help them battle COVID-19, while building the evidence to evaluate which options are effective.”
However, experts feel there is very little evidence to prove that the drugs are effective in treating COVID-19.
In an interview with CBS’ “Face the Nation” on Sunday, former Food and Drug Administration Commissioner Scott Gottlieb said, “We really need to continue to conduct research to find out what works. Right now, there’s no drug that looks like it’s proven so overwhelming in early-stage clinical trials that we can say it’s highly promising.”
© Copyright IBTimes 2024. All rights reserved.











