Infant Medication Recall 2013: Perrigo Acetaminophen Suspension Liquid Recalled Nationwide For Defective Syringe [FULL LIST]

Infants are in danger of potentially overdosing on medication this week after the Perrigo Company issued a voluntary recall for their products following the discovery of defective, unlabeled syringes.
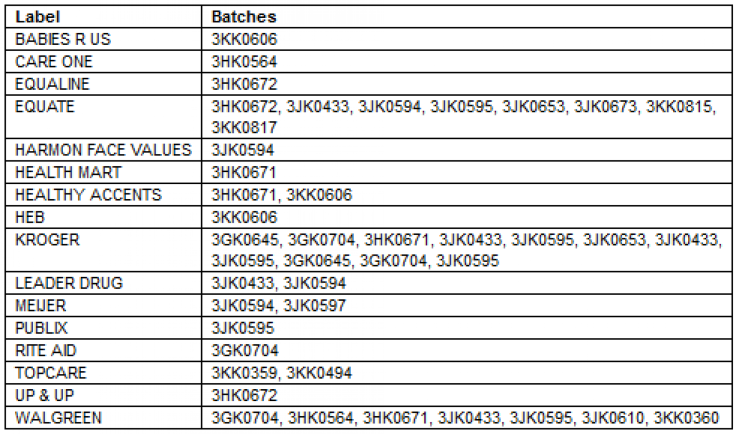
According to a statement released by the Michigan-based global healthcare supplier Friday, 18 batches of their Acetaminophen Infant Suspension Liquid, 160 mg/5mL products are being recalled nationwide for containing a potentially defective oral syringe in their packaging. “The recall is being initiated because of the remote potential that a small number of packages might contain an oral dosing syringe without dose markings,” stated the press release, adding, “The correct syringe should have a white or yellow plunger with specific dose markings for 1.25 mL, 2.5 mL, 3.75 mL, and 5 mL.”
The recalled product, which helps reduce fevers and provides pain relief for infants, children and adults, was sold at the chain retailers Rite Aid, Publix, Walgreens, Kroger and Babies R US. The medication is also distributed by Equate and Up & Up. (To see a full list of involved retails/distributor, click here).
Customers currently in possession of the recalled products are advised to continue use if the dose markings are labeled as shown in the picture above. Perrigo has released a warning for consumers to discontinue use of the unmarked oral syringes to prevent providing inaccurate dosing. According to a statement from Perrigo Chairman, President and CEO Joseph C. Papa, only some applicators are defective, not the infant medication itself.
“Out of an abundance of caution, we are taking this measure to maintain the highest possible product quality standards for our retail customers and consumers,” said Papa. “While we cannot be certain that any of these unmarked dosing devices were released into our customers' supply chains, taking this action is the right thing to do,” he later added.
Perrigo claims no complaints have been reported in connection to the recall. Customers in possession of a recalled products can contact Perrigo’s customer affairs department at: 1-800-719-9260 for more information.
PERRIGO INFANT MEDICINE RECALL:
© Copyright IBTimes 2024. All rights reserved.












