Saturn Moon Titan: NASA Has Explanation For 'Impossible' Cloud Formation

Saturn’s largest moon, Titan, is the only other object in our solar system that has evidence of stable liquid on its surface. The similarities with Earth don’t end there—the moon has a layered atmosphere; contains lakes, rivers and channels (although instead of water, it has pure liquid methane); possesses mountains and possibly volcanoes; and has clouds in its atmosphere. According to the National Aeronautics and Space Administration (NASA), Titan—discovered by Dutch astronomer Christiaan Huygens in 1655—is "one of the most Earth-like worlds we have found to date."
But the stratosphere, the north and south winter poles of the moon, have “impossible” ice clouds whose existence is confusing scientists. The clouds, first spotted decades ago by NASA’s Voyager 1 spacecraft, are made of dicyanoacetylene (C4N2)—the compound partially responsible for giving the moon its brownish-orange hue. What makes their presence so counterintuitive is the fact that the chemical composition of the surrounding stratosphere contains little to no dicyanoacetylene.
Most recently, NASA’s current Cassini-Huygens mission, which has been orbiting Saturn since 2004, found a second example of the cloud. And, similar to the previous discovery, the cloud contains dicyanoacetylene while the surrounding stratosphere has less than one percent of the gas needed for the cloud to exist.
"The appearance of this ice cloud goes against everything we know about the way clouds form on Titan," lead study author Carrie Anderson of NASA's Goddard Space Flight Center said in a statement.
The process of condensation—this takes place when air temperature and pressure can condense vapor into ice—is essential for clouds to exist. "For clouds that condense, this equilibrium is mandatory, like the law of gravity," said Robert Samuelson, an emeritus scientist at Goddard and a co-author of the paper.
On Titan’s atmosphere, the evaporation and condensation of methane results in clouds similar to how the condensation of water creates clouds. But at Titan’s stratosphere, “layers of clouds condense as the global circulation pattern forces warm gases downward at the pole” and the gases “condense as they sink through cooler and cooler layers of the polar stratosphere.” NASA scientists found that Titan’s stratosphere would need “100 times more vapor” to form an ice cloud.
What can explain this phenomenon? Initially, an explanation offered was that the Voyager’s instruments were not sophisticated enough to detect vapor. But with the Cassini spacecraft, which also did not detect vapor, that theory was dropped.
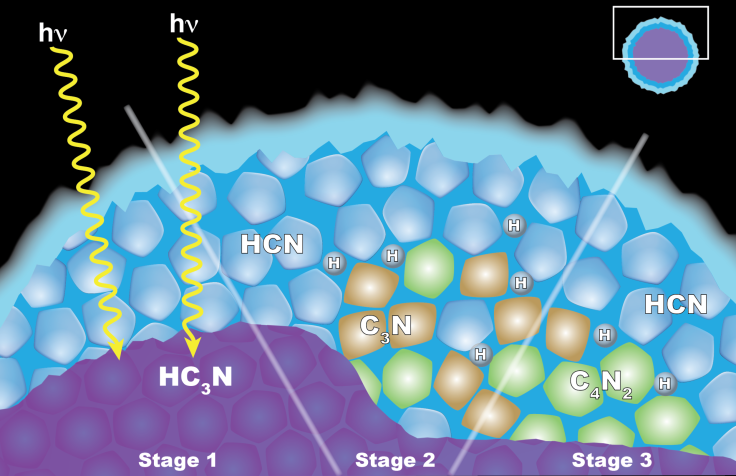
Anderson and her colleagues at Goddard and Caltech have a new explanation: the clouds are not formed by condensation. Instead, they theorize that dicyanoacetylene is formed by reactions taking place inside other ice particles—a process they call “solid-state chemistry.”
“The first step in the proposed process is the formation of ice particles made from the related chemical cyanoacetylene (HC3N),” writes NASA. “As these tiny bits of ice move downward through Titan's stratosphere, they get coated by hydrogen cyanide (HCN). At this stage, the ice particle has a core and a shell comprised of two different chemicals. Occasionally, a photon of ultraviolet light tunnels into the frozen shell and triggers a series of chemical reactions in the ice. These reactions could begin either in the core or within the shell. Both pathways can yield dicyanoacetylene ice and hydrogen as products.”
The team came up with the “solid-state chemistry” theory from clouds in the Earth’s stratosphere that kill the ozone layer. Specifically, chlorine molecules make their way to the stratosphere via ground pollution by sticking to crystals of water ice. Chemical reactions then release the chlorine molecules into the air, which, in turn, destroy the ozone layer.
"The compositions of the polar stratospheres of Titan and Earth could not differ more," said Michael Flasar, CIRS principal investigator at Goddard. "It is amazing to see how well the underlying physics of both atmospheres has led to analogous cloud chemistry."
© Copyright IBTimes 2024. All rights reserved.






















