Schizophrenia Linked To Hyperactivity In Brain's 'Default Mode Network': Mouse Study
Experiments in mice are pointing to an abnormally overactive brain mechanism that may lie at the root of schizophrenia.
Massachusetts Institute of Technology researcher Junghyup Suh and colleagues from the RIKEN-MIT Center for Neural Circuit Genetics and Johns Hopkins University were recording activity in the hippocampuses of mice as they ran through a maze. Some of the lab mice were genetically tuned to exhibit symptoms similar to schizophrenia. These mice lack the brain protein calcineurin, and mutations in the gene that produces this protein have been found in some human schizophrenia patients. The mice that have calcineurin “knocked out” of their systems tend to have faulty short-term memory, abnormal social behavior and attention deficits.
In a paper appearing in the journal Neuron on Wednesday, Suh and colleagues describe how the neural activity differed between the hippocampuses of the normal mice and the schizophrenic-like mice.
“The hippocampus is important because it’s part of the so-called default mode network,” Suh explained in a phone interview.
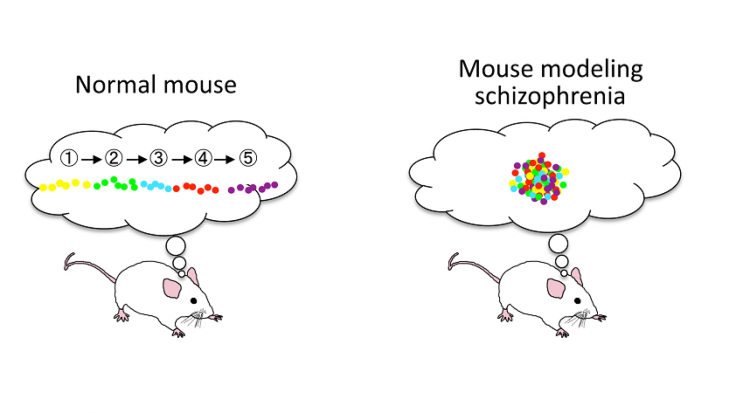
This default mode network consists of communication links between the hippocampus, prefrontal cortex, and other brain regions. It fires up when an organism is resting (but not asleep) and not focused on tasks. The network has links to daydreaming, memories – and, when overactive, it’s thought to play a role in schizophrenia.
Normally, when a mouse takes breaks from running around a maze, certain cells in the hippocampus activate as the default mode network fires up and starts processing the recent activity. The cells light up at a moderate level, in a certain sequence. But Suh and colleagues found that in the schizophrenic-like mice, this activity was kicked into overdrive. And in the schizophrenic-like mice, the hippocampus cells seemed to light up all at once instead of in an orderly fashion. This suggests that the mouse is undergoing some kind of disorganized thinking process that’s preventing it from “replaying” the maze route it just traveled.
It’s possible that calcineurin, the enzyme that the schizophrenic-like mice lack, may be important in dialing down the activity across the neurons of the hippocampus. Without its mediating influence, mice and men might be caught in the grip of abnormal brain activity. Could restoring the protein to mice mitigate their schizophrenia-like systems? Suh doesn’t know for sure, but he says it’s definitely a consideration for future research.
Suh and colleagues say their findings could help unify several seemingly disparate aspects of schizophrenia. Psychosis and disordered thinking seen in schizophrenia patients may stem from abnormal memory reactivations in the cortex and hippocampus, thanks to this hyperactivity.
"Our study provides new insight into what underlies schizophrenia's disordered thinking and zeroes in on a new target for future investigation into the neural basis of a cognitive disorder that affects more than 1 percent of the world's population," senior author Susumu Tonegawa said in a statement Wednesday.
SOURCE: Suh et al. “Impaired hippocampal ripple-associated replay in a mouse model of schizophrenia.” Neuron, 16 October 2013.
© Copyright IBTimes 2024. All rights reserved.











