Grandmother, 90, Gets First Covid Jab As UK Starts Vaccine Drive
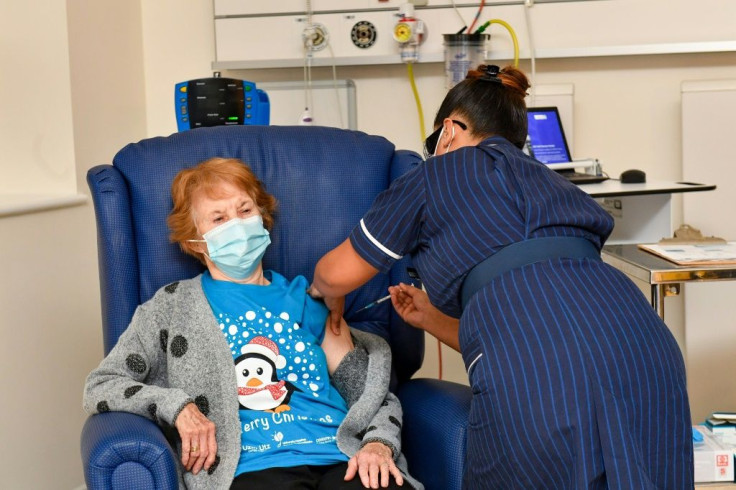
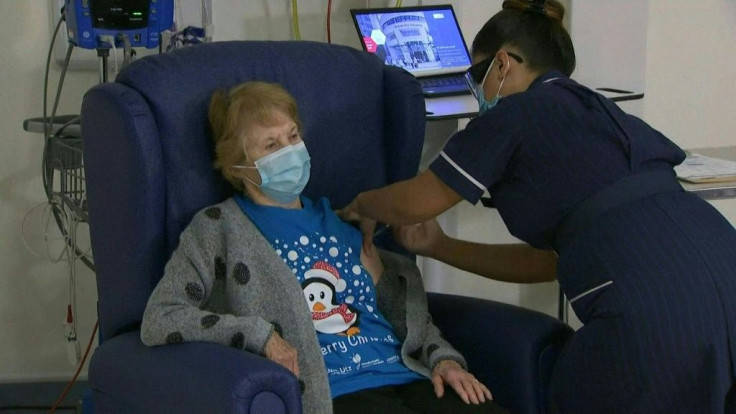
Nurses cheered on Tuesday as a 90-year-old grandmother in Britain became the first person in the Western world to receive an approved vaccine against Covid-19, at the start of a marathon campaign health officials hope heralds a fight-back against the pandemic.
Margaret Keenan, who turns 91 next week, called the jab she received at a hospital in Coventry, central England, "the best early birthday present".
"My advice to anyone offered the vaccine is to take it. If I can have it at 90 then you can have it too," said the former jewellery shop worker who has two children and four grandchildren.

"I feel so privileged to be the first person vaccinated against Covid-19."
Regulators in Britain -- one of the worst-affected countries with more than 61,000 deaths from 1.6 million cases -- became the first to approve the Pfizer-BioNTech vaccine for general use last week.
Keenan, wearing a Christmas t-shirt, received the jab in front of cameras at 0631 GMT, and received a guard of honour from clapping medics as she was wheeled back to her bed.
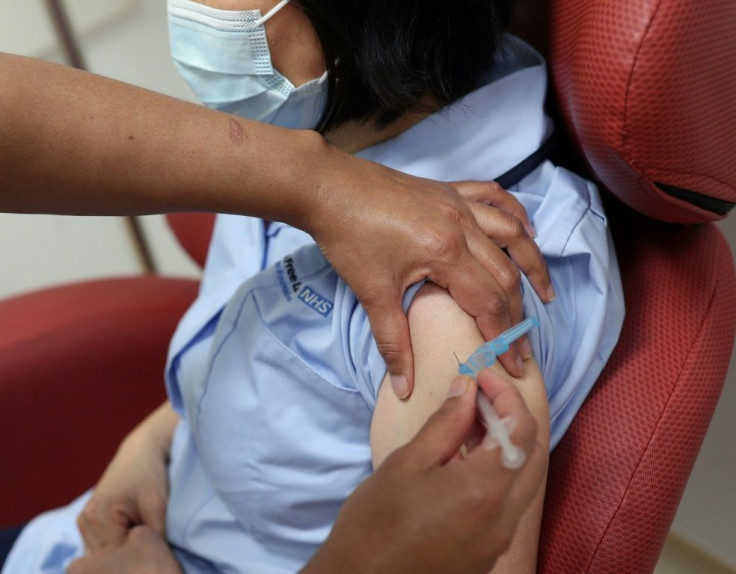
She was followed by a man called William Shakespeare who, like the world-famous playwright, is from Warwickshire.
May Parsons, a nurse originally from the Philippines who has worked for Britain's state-run National Health Service (NHS) for 23 years, called it a "huge honour" to administer the jab to Keenan.
The over-80s, care home workers and at-risk health and social care staff are first in line to get the jab, on what has been dubbed "V-Day". A second jab is required after 21 days.
Prime Minister Boris Johnson, who spent days in intensive care with Covid-19 earlier this year, called it "a tremendous shot in the arm for the entire nation".
But with most people not expected to get vaccinated until early 2021, he said the public still needed to be careful to stop the spread of the virus.
"We can't afford to relax," he said on a visit to a central London hospital.
The UK has ordered 40 million doses of the jab -- enough to vaccinate 20 million people -- with 800,000 in the first batch.
Up to four million doses are expected by the end of December.
The public has largely welcomed the rapid approval of the vaccine but ministers and health professionals are aware they still need to combat mistrust.
Reports suggest Queen Elizabeth II, who at 94 is among those first in the line for the vaccination because of her age, could front a public awareness campaign urging compliance.
The government said it would hand out vaccine cards to remind people to get the booster after three weeks, but insisted it was not introducing immunity certificates or making jabs compulsory.
"It's like any other injection," said care worker Stephen Pearson Perry after receiving his jab in Preston, northwest England, adding he was not expecting any side effects.
In Edinburgh, project engineer Sean Wilson, 36, said on balance, vaccination would be beneficial. "I think everybody should go for it," he told AFP.
"What's the worst that could happen? It couldn't get any worse really than what it is now."
Health officials have already run into a logistical headache about how to administer the vaccine to vulnerable care home residents.
The vaccine needs to be stored at -70 degrees Celsius (-94 Fahrenheit), leaving hospitals and other medical hubs as the only places able to deal with such ultra-low temperatures.
Concerns have also been raised about potential disruption to supply when Britain leaves the European Union's single market and customs union, as the Pfizer-BioNTech drug is made in Belgium.
The bulk of Britain's vaccine requirements are expected to be met by a jab developed by AstraZeneca and the University of Oxford, which is awaiting regulatory approval.
The government has ordered an initial 100 million doses of the drug, which is cheaper to manufacture and easier to store and transport.
© Copyright AFP {{Year}}. All rights reserved.





















