New Guidelines From The CDC For Coronavirus Testing Bring More Meaningful Results. What To Know About Wednesday’s Pandemic Developments
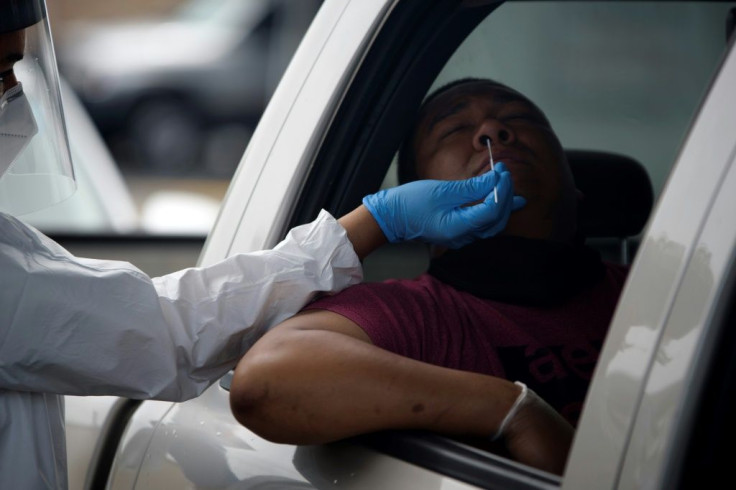
The Centers for Disease Control and Prevention changed its guidelines for coronavirus testing, which Admiral Brett P. Giroir, assistant secretary for Health at the U.S. Department of Health and Human Services, told reporters would allow for more meaningful tests.
The CDC said it no longer requires asymptomatic people to get a coronavirus test even if they have come in contact with a person that has COVID-19. Now the CDC says asymptomatic people should not "necessarily” be tested unless a health official suggests otherwise.
The CDC is instead focusing its attention on people who are more vulnerable to the coronavirus, which Giroir said was based on advice and evidence from medical experts such as Dr. Anthony Fauci, the nation's top infectious disease expert; Deborah Birx, who is coordinating the administration response to the pandemic, and Stephen Hahn, head of the Food and Drug Administration.
Giroir also said negative test results can provide a false sense of security. It doesn’t mean that someone can go visit grandma and expect not to infect her, he said.
Giroir said while the new guidelines recommend against some testing, the number of tests likely will not decrease. Rather ,Giroir said he expects the number of tests to increase as more people return to work and school. The U.S. has administered 79.6 million coronavirus tests to date, he said.
The CDC does recommend that people who have no symptoms of the coronavirus “realize that you can be infected and spread the virus but feel well and have no symptoms.”
“You do not necessarily need a test unless you are a vulnerable individual or your healthcare provider or state or local public health officials recommend you take one,” the agency said on its website, adding, “If you are tested, you should self-isolate at home until your test results are known, and then adhere to your healthcare provider’s advice.”
The new testing guidelines from the CDC come as an official told The New York Times the Trump administration pressured the agency to limit testing criteria. Trump has suggested testing should be reduced to stem the burgeoning number of coronavirus cases, which was reflecting poorly on the U.S.
Giroir denied politics influenced the new guidelines, saying the decision was based on the growing body of scientific evidence.
“There was no weight on the scales by the president or the vice president or [Health] Secretary [Alex] Azar,” Giroir said. “This was a product produced by the scientific and medical people that was discussed extensively at the task force.”
In other coronavirus news:
- The Florida Department of Health has released data that showed nearly 9,000 children have been infected with COVID-19 in the last 15 days. As much as 80% of new cases were children ages 5 to 17. Florida also saw hospitalizations of children rise from 436 to 603 over the same time period with one child death, bringing the total number of children’s deaths to eight.
- Both Ford and GM are delaying when their office employees can come back to work after working remotely since March. Ford said it will be at least January before it makes a decision while GM said it will reevaluate the situation in October, The Detroit News reported. GM said when it does decide to bring workers back, it will use a phased approach. Fiat Chrysler has not indicated when it will allow workers to return to its offices.
- Moderna announced its COVID-19 vaccine showed promising results in elderly adults in early clinical trials. Participants of the trial were 56 and older and received two 100 microgram doses of the vaccine 28 days apart. Moderna said the patients produced neutralizing antibodies, which may build up immunity to the virus. Patients also showed good tolerability to the vaccine and minimal side effects, Moderna said.
- VBI Vaccines announced it selected two vaccine candidates to move to Phase 1/2 human trials after three were tested in mouse studies. The trials will begin at the end of 2020 and are subject to regulatory approval. Results from the mouse study indicated the trial program could be administered as a one-dose vaccine regimen, VBI said.
- The Department of Health and Human Services announced the release of 1.5 million N95 respirators from the Strategic National Stockpile to 3,336 nursing home facilitiesthat have reported only enough supplies for up to three days of operation. Each nursing home will receive a seven day supply, HHS said.
- MaskMarket.com has launched an LED Smart Mask that will allow wearers of the face mask to show off an electronic smile. The mask, which is sound activated, lights up and simulates facial expressions. The mask has a light board inside that syncs with the wearer’s voice and is activated through sound. The mask costs $47.95, plus shipping and handling.
- Globally there are more than 23.9 million positive cases of the coronavirus, with COVID-19 deaths exceeding 821,000, according to Johns Hopkins University. The U.S. still leads the world in coronavirus cases with more than 5.8 million confirmed cases and 179,000. The next two leading countries in positive coronavirus cases are Brazil and India. COVID-19 death counts behind the U.S. include Brazil, Mexico and India.
© Copyright IBTimes 2025. All rights reserved.





















