Oxygen On Mars, 4 Billion Years Ago: Geological Puzzle Gives Clues To Ancient Atmosphere
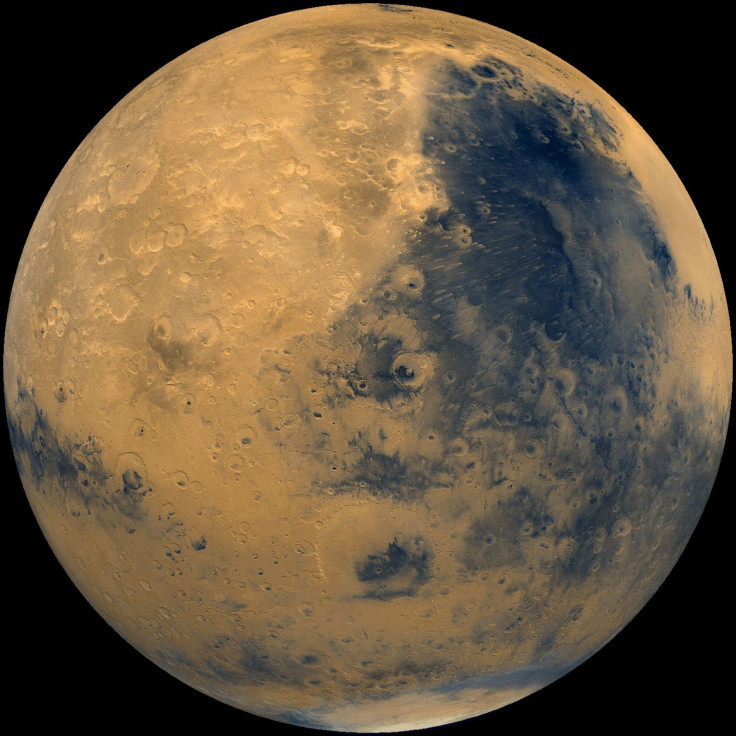
Billions of years ago, ancient Mars might have had an oxygen-rich atmosphere as well as watery seas.
In a study published on Wednesday in the journal Nature, Oxford University scientists say the best way to explain some of the geological differences between meteorites from Mars that have landed on Earth and rocks still on the surface of the Red Planet is the existence of a Martian atmosphere rich in oxygen about 4 billion years ago. At that time in its history, the Red Planet was under heavy bombardment by asteroids and meteorites, undergoing significant volcanic activity, and possibly had liquid water seas on its surface. All of this was going on about the same time that Earth’s earliest life forms are thought to have arisen but long before our planet had an oxygen-heavy atmosphere.
The clues that point to oxygen on Mars lie at the heart of a geological puzzle. Rocks inside Mars’ Gusev crater examined by NASA’s Spirit rover are about 3.7 billion years old and contain about five times as much nickel as Martian-derived meteorites, which are much younger, hailing from anywhere between 1.4 billion and 180 million years ago.
That difference in nickel concentration was a flag that alerted Oxford earth scientist Bernard Wood and his team that the two sets of rocks differed by more than just age.
“Nickel likes to be associated with sulfur,” Wood wrote in an email. In the low-oxygen volcanic furnace that forged the meteorites, melting nickel would have been trapped inside sulfide minerals, leaving those rocks low in nickel. But when material is melted in a more oxygen-rich environment, the sulfur dissolves.
“Hence the surface rocks, formed under more oxygen-rich conditions in Mars' interior, are richer in nickel,” Wood said.
Wood and his colleagues think that much of the material on the surface of Mars was oxidized very early on in the history of the planet, drawn underground in a process called subduction, then expelled back to the surface in volcanic eruptions. The meteorites, on the other hand, are the product of much more recent volcanic forces and come from deeper within Mars and were thus unaffected by the same processes.
Oxygen on Mars “would originally have been produced by the breakdown of water vapor in Mars' atmosphere,” Wood said. “This produces hydrogen and oxygen and some of the hydrogen would be completely lost to space, leaving some oxygen in the atmosphere.”
On the surface, the oxygen would be consumed as it oxidized iron, turning it into the compound iron oxide (one form of which is rust). That process is what gave Mars its iconic red hue. But just how oxygen-rich was ancient Mars?
“Unfortunately, we can't be sure, because we don't need very large concentrations of oxygen to produce the oxidized rocks at Mars' surface,” Wood said. “All we know is that oxygen was available.”
So where did all that oxygen on Mars go? Basically, the well dried up. Even though atmospheric oxygen was being consumed through oxidation on the surface, it was replenished as water vapor was added to the atmosphere by the steam from volcanic eruptions.
But as the planet cooled and became less volcanically active, “the water vapor in the atmosphere declined with time and the oxygen was no longer being replaced rapidly enough to maintain its concentration in the atmosphere,” Wood said. “Hence Mars ‘lost’ its oxygen.”
SOURCE: Tuff et al. “Volcanism on Mars controlled by early oxidation of the upper mantle.” Nature published online 19 June 2013.
© Copyright IBTimes 2025. All rights reserved.





















