Scientists Observe Quantum States Of Molecular Ions Using Laser-Cooled Atomic Ions

Storing information using quantum properties of subatomic particles and atoms — the key prerequisite for building a quantum computer — is extremely hard, primarily because these properties are typically very fragile. Although physicists have, in recent years, succeeded in effectively demonstrating quantum control of individual atoms, they face an added level of complexity when it comes to doing the same with molecules.
Now, in a study published Wednesday in the journal Nature, a team of physicists from the National Institute of Standards and Technology (NIST) in Boulder, Colorado, describe a successful attempt at using previously known techniques to cool and control molecular ions.
Read: Quantum Computers Face Off In Benchmark Test
The successful experiment, which involved using a laser to gently probe molecular ions to detect their quantum states indirectly, could pave the way for the creation of much more complex and computationally faster quantum computers.
“We developed methods that are applicable to many types of molecules. Whatever trick you can play with atomic ions is now within reach with molecular ions. Now the molecule will ‘listen’ to you—asking, in effect, ‘What do you want me to do?’” NIST physicist James Chin-wen Chou, the lead author of the study, said in a statement. “This is comparable to when scientists could first laser cool and trap atoms, opening the floodgates to applications in precision metrology and information processing. It’s our dream to achieve all these things with molecules.”
Of course, controlling and observing quantum states is much harder for molecules than it is for atoms, primarily because molecules consist of many combinations of atoms. For instance, the calcium hydride molecular ion (made of one calcium ion and one hydrogen ion bonded together) used by NIST in their experiments has 100 possible rotational states.
While this property ensures that molecular ions offer more options than atomic ions insofar as storing and converting quantum information is concerned, it also makes observing transitions in their quantum states without destroying them an extremely daunting task.
In order to do so, the researchers first confined a calcium hydride ion and a calcium atomic ion in an electromagnetic trap. Because of their physical proximity in the ion trap and the repulsive interaction of their electrical charges, the two ions developed a pendulum-like shared motion. The researchers then used a laser to cool the atomic ion, which, in turn, caused the molecule to drop to its lowest-energy state.
Once this was achieved, the researchers used tuned pulses of infrared laser light that targeted only one unique transition out of the 100 possible ones in the molecule’s rotation. When the transition to the targeted state occurred, some energy was transferred to the ions’ shared motion, and they started moving again. Another laser pulse was then used to convert the change in the shared motion into a change in the atomic ion’s internal energy level — which was manifested in the form of light scattered by the atomic ion.
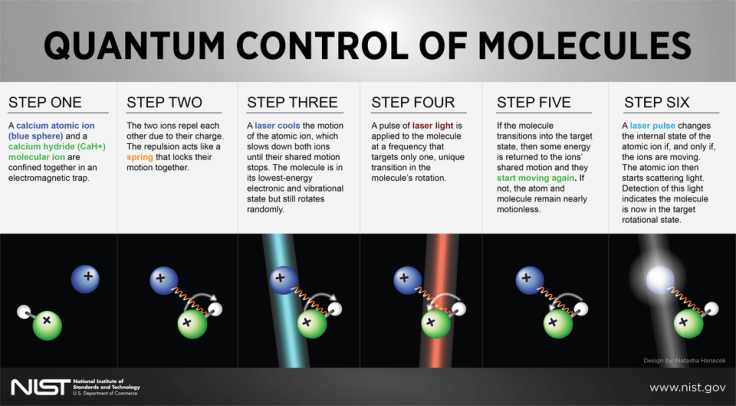
“The molecule only jiggles if it is in the right state. The atom feels that jiggle and can transfer the jiggle into a light signal we can pick up. This is like Braille, which allows people to feel what is written instead of seeing it. We feel the state of the molecule instead of seeing it and the atomic ion is our microscopic finger that allows us to do that,” the study’s senior author Dietrich Leibfried, also from NIST, said in the statement. “Moreover, the method should be applicable to a large group of molecules without changing the setup. This is part of NIST's basic mission, to develop precision measurement tools that maybe other people can use in their work.”
© Copyright IBTimes 2025. All rights reserved.





















