Infant Motrin Recall 2013: One Month Later, Drops Still Being Recalled For Containing Plastic Particles [LIST]
After being recalled almost a month ago, a recall of nearly 200,000 bottles of infant Motrin for possible contamination of plastic is still in effect, the company said Monday.
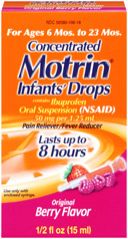
Last month, a recall was issued for an estimated 200,000, or three lots, of "Concentrated MOTRIN® Infants’ Drops Original Berry Flavor" products sold in the U.S. in the 1/2 fl. oz. size. Motrin, which is manufactured by The McNeil Consumer Healthcare Division issued the recall in association with the U.S. Food & Drug Administration (FDA) for possibly containing plastic particles.
However, nearly 30 days later after the voluntary recall was announced and the product being pulled from store shelves, the recall is still in effect. The recall was first announced on Sept. 9.
"The potential for adverse medical events related to the reason for this recall is not likely," the company said in a news release Sunday.
Motrin, which is owned by New Jersey-based Johnson & Jonhson, issued the recall after three lots of were found to contain tiny plastic particles -- approximately 1 mm in size “about the size of a poppy seed” – in the product. Later, it was found that the plastic particles actually came from a third party supplier of ibuprofen, the active ingredient in the infant Motrin.
While the company claims there are no “adverse medical events related to this recall,” McNeil asked consumers to stop using the product and dispose of any of the recalled product.
Below is a list and information of the infant Motrin affected under the recall. Consumers affected by the recalled products can received a refund or product coupon by calling 1-877-414-7709 or submitting a request online.
MOTRIN INFANTS’ DROPS 2013 RECALL:
Product: Concentrated MOTRIN® Infants’ Drops Original Berry Flavor 1/2 fl. oz. bottles
National Drug Code (NDC): 50580-100-18
Lot codes: DCB3T01, DDB4R01, DDB4S01
UPC Code: 300450524157
Case UPC Code: 30300450524158
© Copyright IBTimes 2025. All rights reserved.






















