Myths About The Sun That Turned Out To Be Wrong


This question originally appeared on Quora. Answer by Jack Fraser.
Well, a lot of people used to think (and many laypeople still do) that the sun is a big ol’ ball of fire.
I mean — it’s not the most absurd assumption to make, I mean, look at it!

Big, orange-y/yellow ball of brightness?
What could it possibly be if not fire?

You can use the sun to start fires:

It’s hot, and can burn you:
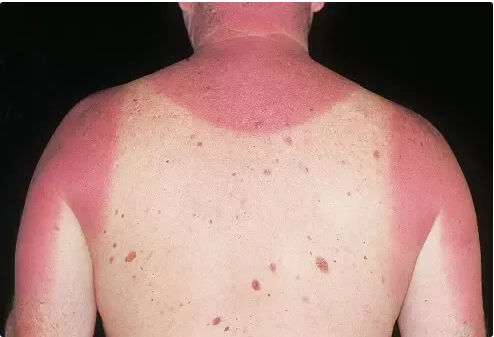
The fact that the sun was made of fire was taken as blindingly obvious for many hundreds of years.
There were some hints that this couldn’t be the case — for example, early spectrometry (looking at the wavelengths of light visible in the sun) showed that we could identify the elements in the sun, by looking at the absorption lines:
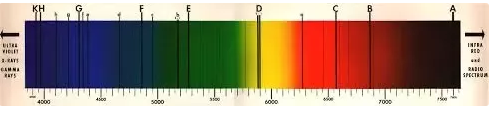
This showed that the Sun was primarily composed of hydrogen — which sounds like it should be good for the whole “fireball” theory — hydrogen is, after all, rather infamously flammable:

But — there was one rather huge problem.
The sun contains no Oxygen.
They already knew (thanks to Priestly, Lavoiser etc.) that Oxygen was the vital component of combustion — no Oxygen, no fire.
So if the sun had no Oxygen, how could it be on fire?
The usual explanation for this was that spectrometry could only tell you about the elements on the surface of the sun — not what was inside it. Therefore the oxygen was probably inside the core of the sun, sustaining the combustion reaction.
The spectrometry results remained very controversial - most people disregarded them entirely, and the idea that the sun was mostly hydrogen was not accepted for a long time.
Then came the quantum revolution — we learned loads, and loads about subatomic physics. We learned about the atomic nucleus and the “weird” world of quantum behaviour.
A physicist by the name of George Gamow then produced a formalism for describing alpha decay using quantum tunneling (first noted by Friedrich Hund)— which allowed atoms to cross the classically impenetrable Coulomb barrier.
Two people (Robert d'Escourt Atkinson and Fritz Houtermans) then used this work to predict a phenomenon called nuclear fusion.
They predicted that, given suitable initial conditions, light nuclei (such as hydrogen and helium) could undergo this process, and fuse to form heavier nuclei — in the process, producing huge amounts of energy.
They therefore predicted that our sun was not a gigantic fireball — but was instead a very dense (mostly) hydrogen plasma, which produced the suitable conditions for nuclear fusion to take place.
This idea remained controversial — it still wasn’t wholly accepted that the sun was mostly hydrogen.
However, Hans Bethe then worked out some of the more complex nuances of the hydrogen fusion chain, and the evidence of the sun’s composition continued to mount up.
Eventually, the conclusion was inescapable:
The sun is not a gigantic ball of fire, as was originally believed to be true. It is actually a very large ball of hydrogen plasma, which produces energy by fusing light nuclei into heavier nuclei.



















