uDisco: 3D Imaging That Makes Body Tissue Transparent May Help Map Human Brain
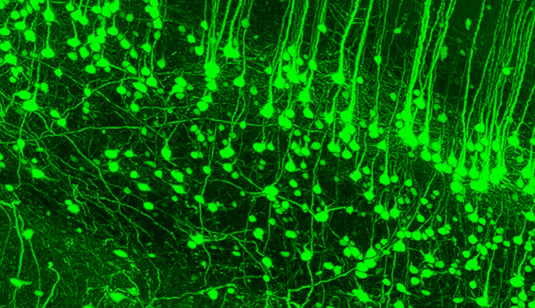
Most medical research to understand diseases uses lab mice: take a piece of thinly sliced tissue and study it under a powerful microscope. While the method has proven largely effective over the past many decades, it has associated costs and significantly, it is also extremely difficult to create complete 3D models of an organ or organism using individual slices of tissue.
To address that problem, particularly in the context of the human brain, Ali Ertürk and his colleagues from the Institute for Stroke and Dementia Research, Ludwig Maximilians University Medical Center in Munich, Germany, have come up with a novel method they call “ultimate 3D imaging of solvent cleared organs” or uDisco.
On its website, the Institute says its “laboratory is interested in understanding key mechanisms leading to neurodegeneration after acute brain injuries: stroke and trauma.” And citing “the difficulty to accurately analyze long neuronal connections in the brain,” it speaks of the “cutting-edge imaging techniques including high-resolution 3D imaging of the entire brain that we recently developed.”
The uDisco process, using a rodent, is explained in a paper published in the journal Nature Methods. The method “preserves fluorescent proteins over months and renders intact organs and rodent bodies transparent while reducing their size up to 65%. We used uDISCO to image neuronal connections and vasculature from head to toe over 7 cm and to perform unbiased screening of transplanted stem cells within the entire body of adult mice.”
Shrinking the body being studied is important because microscope designs have their own limitations. Furthermore, thick tissue tends to scatter light, which is another way of saying that it is the thickness of tissue that makes it opaque. Ertürk’s team first put a dead mouse in alcohol to dehydrate the body and then soaked it in an organic solvent to remove all the fat from it. An added fluorescent protein makes specific parts of the body glow, making it easier to study later.
In about four days, the mouse becomes transparent.
Talking about the possible applications of the method in studying brain disorders, and the current ways to study them — which involve examining slices of brain tissue under a miscroscope — Ertürk said: “That is not a good way to study neurons because if you slice the brain, you slice the network. The best way to look at it is to look at the entire organism, not only the brain lesion but beyond that. We need to see the whole picture.”
© Copyright IBTimes 2024. All rights reserved.





















