A Wire Only 3 Atoms Wide, Made Using Really Tiny Diamond Pieces
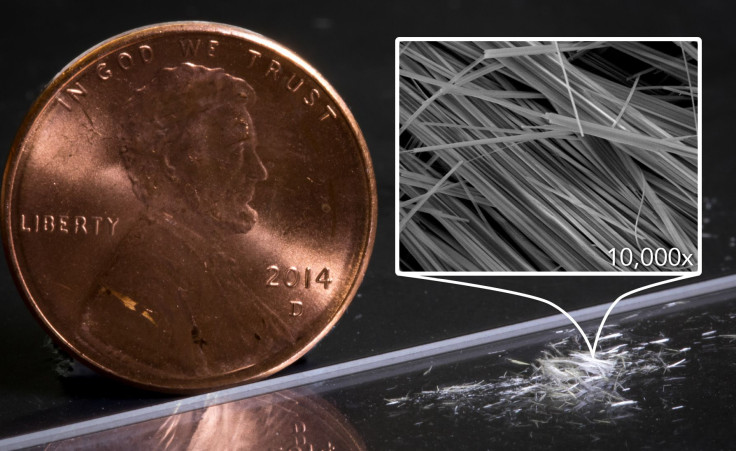
The thinnest wires today are not the sort of loopy cables that connect the power sockets in the walls, but are instead simply printed on circuit boards that are inside everything from your 65” flat-screen TV to the tiniest chip inside your smallest gadget. At just a few tens of micrometers wide, they are about the same width as human hair.
Now imagine a wire that is only three atoms wide, or about 1,000 times narrower than human hair. If it helps, think of the difference between a hair strand and a pencil, and apply that same difference in scale to the imaginary wire and the hair strand. Existing as practically a one-dimensional material, such a wire would have extraordinary properties.
Scientists from Stanford University and the Department of Energy’s SLAC National Accelerator Laboratory have created such a wire using diamondoids, the smallest possible pieces of diamond. Using a combination of copper and sulfur known as a chalcogenide for a superconducting core and an insulating shell made of diamondoids, the nanowire assembles itself.
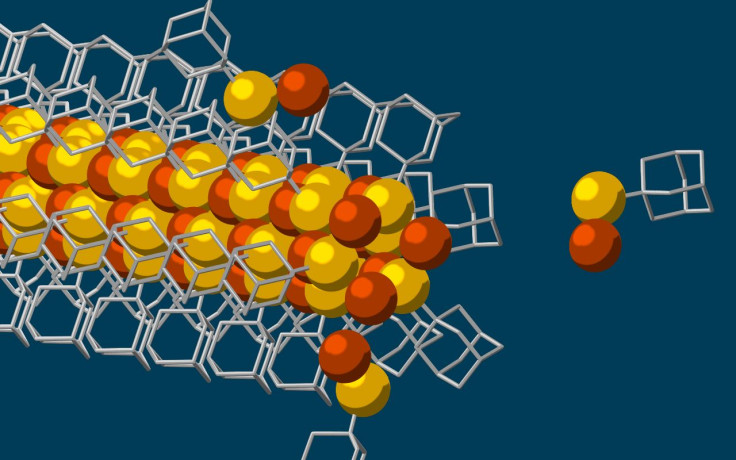
Hao Yan, a Stanford postdoctoral researcher and lead author of a study on the subject, said in a statement: “What we have shown here is that we can make tiny, conductive wires of the smallest possible size that essentially assemble themselves. The process is a simple, one-pot synthesis. You dump the ingredients together and you can get results in half an hour. It’s almost as if the diamondoids know where they want to go.”
Diamondoids are essentially “tiny, interlocking cages of carbon and hydrogen” that are found naturally in petroleum fluids. These microscopic particles are strongly attracted to each other, which allows them to form clumps that are visible to the naked eye. Taking the smallest possible diamondoids — containing only 10 carbon atoms — scientists attached a sulfur atom to each, which in turn attached with a single copper ion. Different diamondoid particles, each with their sulfur and copper bonds, linked up due to their inherent attraction, making long thin chains, resulting in the creation of the nanowire.
“Much like LEGO blocks, they only fit together in certain ways that are determined by their size and shape. The copper and sulfur atoms of each building block wound up in the middle, forming the conductive core of the wire, and the bulkier diamondoids wound up on the outside, forming the insulating shell,” Stanford graduate student Fei Hua Li, who played a critical role in synthesizing the tiny wires and figuring out how they grew, said in the statement.

Using their method of self-assembly, the researchers achieved precision and control over the material at an atomic level, making it potentially useful in a wide range of applications, “including fabrics that generate electricity, optoelectronic devices that employ both electricity and light, and superconducting materials that conduct electricity without any loss.”
The study was published Monday in the journal Nature Materials under the title “Hybrid metal–organic chalcogenide nanowires with electrically conductive inorganic core through diamondoid-directed assembly.”
© Copyright IBTimes 2024. All rights reserved.











