Chloroquine Coronavirus Cure? Not So Fast, Chinese Doctors Say
KEY POINTS
- The U.S. has a stockpile of 29 million doses of hydroxychloriquine and President Trump has been pushing its use
- Chinese doctors say the Kaletra, an anti-HIV drug, is more promising
- The FDA has authorized the emergency use of two forms of chloroquine for use in fighting COVID-19
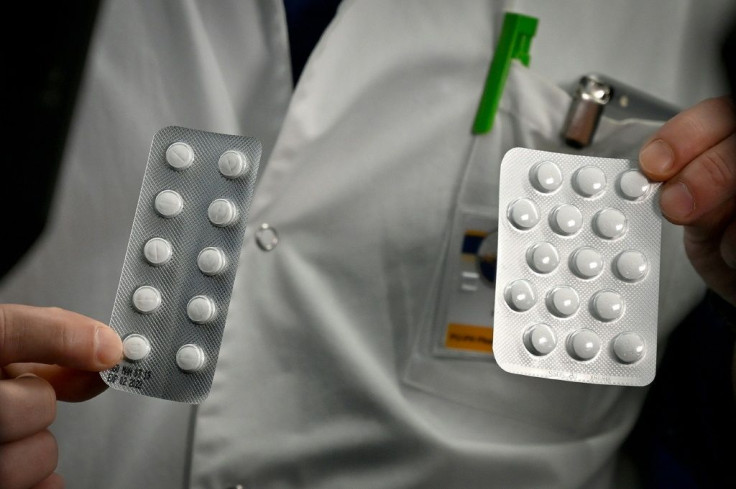
Chloroquine, the anti-malaria drug touted by President Trump as a possible treatment for COVID-19, may not be effective against the coronavirus after all. Chinese doctors, who have been using the drug for months, said Thursday there’s no clear evidence chloroquine has had much impact.
The Wall Street Journal reported it conducted interviews set up by Chinese authorities in the industrial city of Wuhan where the pandemic began late last year. The interviews came one day after the government eased its lockdown on Wuhan.
The doctors told the Journal chloroquine should be studied further, but there’s evidence of chloroquine’s efficacy against the coronavirus is unclear, with some patients still testing positive as many as 10 days into treatment.
“Some patients took it by themselves, and after taking it, there were good and bad” outcomes, Zhang Dingyu, the head of Wuhan’s Jinyintan Hospital, told the Journal. “There’s no scientific conclusion.”
“You can’t see any difference between it and other treatments,” Zhang Junjian, a Wuhan field hospital doctor, told the Journal. He said 20 to 30 patients had been treated with the drug, which can be fatal at certain dosages.
The doctors said, however, the antiviral HIV drug Kaletra, produced by AbbVie, has shown some promise. The drug works by blocking enzymes some viruses need to replicate. Zhang Dingyu said Kaletra, combined with the antibacterial bismuth subcitrate potassium, produced dramatic changes in patients’ lungs. He said he would recommend its usage within three to five days of becoming ill.
Trump, who has a small financial investment in one of the firms that produces hydroxychloroquine, has touted chloroquine’s use at his daily press conferences, and the Food and Drug Administration authorized two forms of the drug for COVID-19 treatment, as well as blood plasma gathered from patients who have recovered.
The U.S. has a stockpile of 29 million doses of hydoroxychloroquine. Despite the lack of evidence it is effective against COVID-19, Trump keeps asking, “What do you have to lose?” He also maintains the drug is safe despite warnings from medical experts about harmful side effects, including liver failure, hearing loss and muscle paralysis. A 2018 review also found neurological damage from the drug.
© Copyright IBTimes 2025. All rights reserved.






















