NASA?s Microgravity Salmonella Vaccine Investigation Revolutionizes Space Microbiology
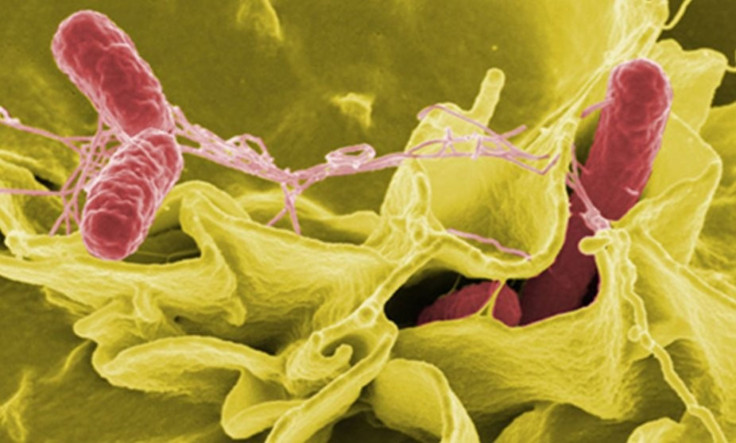
Two Arizona State University research teams have partnered with NASA's Johnson Space Center to strengthen the realms of space microbiology.
The combined team has launched a Recombinant Attenuated Salmonella Vaccine or RASV investigation to the International Space Station aboard the STS-135. The resultant samples of this spaceflight cultured RASV strain has returned to Earth on July 21, 2011 for comparison with a second control sample developed on the ground.
The bacteria, Streptococcus pneumonia, cause a number of life-threatening diseases in new-born and the elderly like pneumonia, meningitis, and bacteremia. Annually, it is estimated that this strain of bacteria is responsible for more than 10 million deaths.
The vaccine samples that were carried aboard the STS-135 are a genetically altered variant of Salmonella that carries a protective antigen against Streptococcus pneumonia. The addition of this strain stimulates a protective immune response without causing any disease itself.
"We have the opportunity to utilize spaceflight as a unique research and development platform for novel applications with potential to help fight a globally devastating disease," stated Cheryl Nickerson, one of the research group's team lead.
Nickerson, along with Roy Curtiss III from the second Arizona research team designed the RASV experiment to use the unique microgravity environment of the space station National Laboratory to increase the vaccine's anti-pneumococcal effectiveness by maximizing its ability to induce a protective immune response.
The oral vaccine, RASV has a number of advantages over vaccines delivered by a needle. This includes activation of an additional arm of the immune system that cannot be engaged by vaccines that are administered as a shot.
Researchers will now evaluate the space-flown strain against the control sample for its ability to protect against pneumococcal infection and changes in gene expression.
Molecular targets identified from this work hold promise for translation to develop new and improve existing anti-pneumococcal RASVs to prevent disease for the general public. Moreover, because RASVs can be produced against a wide variety of human pathogens, the outcome of this study could influence the development of vaccines against many other diseases in addition to pneumonia.
© Copyright IBTimes 2025. All rights reserved.





















