Oil And Water Do Mix: MIT Team Makes Stable Emulsions

Water and oil have been foes forever. It is widely established these two liquids do not mix because of different densities. But, mixing water and oil can have interesting applications in new drug-delivery systems and food-processing methods. A team of researchers at Massachusetts Institute of Technology (MIT) might have successfully mixed the two together in a process that keeps the mixture stable for months and does not even require shaking.
There are processes that can create emulsions. Just vigorously shaking a mixture of water and oil will do the trick. But, the problem with this is the particles simply break into smaller particles and are then suspended. They eventually collate and separate from each other. On an industrial scale, vigorous mixing of the two will be an energy intensive process. To counter this, the team has figured out a way to keep the emulsion stable for months and the process requires no energy. Instead of shaking or blasting a mixture with sound waves, like industries currently do, they used a very simple physical phenomenon called condensation.
The oil needs to be prepared for the emulsion using a surfactant. This soap-like substance will easily bind both oil and water molecules together, thereby eliminating the shaking. Then the oil is placed in a container with humid air. When this container is cooled, water droplets in the air condense and form uniform, microscopic droplets that spread through the oil evenly.
The study, conducted by former postdoc Sushant Anand and associate professor Kripa Varanasi, was published in the journal Nature Communications.
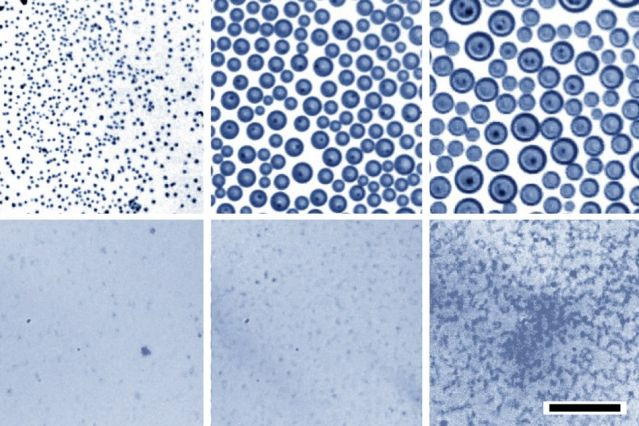
“The key to overcoming that separation is to have really small, nanoscale droplets,” graduate student Ingrid Guha, a member of the study, said in a press release on the MIT website. “When the drops are small, gravity can’t overcome them,” and they can remain suspended indefinitely.
Using a trial and error method, the team got the best oil and surfactant mixture for efficient mixing. The water droplets mix with the oil because they are reduced to nanoscale sizes. These tiny particles are oblivious to gravity. This stops the particles from collecting in the emulsion, making it stable for months.
“If you get the chemistry just right, you can get just the right dispersion,” Guha added.
The key, according to the team, is the proportions of oil and surfactant used.
The stable emulsion was found intact after months, proving this method would be energy and cost efficient.
“The droplets stay so small that they’re hard to see even under a microscope,” Guha added in the release.
The condensation method used in the study follows a bottom-up approach, according to the team. Instead of mechanically shaking or using sound waves, the condensed droplets start-off very small. Therefore, they do not need the extra energy to break them down.
“By cloaking the freshly condensed nanoscale water droplets with oil, we are taking advantage of the inherent nature of phase-change and spreading phenomena,” associate professor Varanasi said in the release.
“Our bottom-up approach of creating nanoscale emulsions is highly scalable owing to the simplicity of the process,” Sushant Anand said. “We have uncovered many new phenomena during this work. We have found how the presence of surfactant can change the oil and water interactions under such conditions, promoting oil spreading on water droplets and stabilizing them at the nanoscale.”
According to the team the discovery is very important because the food and pharmaceutical industry will always have an expiration date and this is mostly because of the instability of the emulsions. This process can be implemented to a wide range of un-mixable liquids, making it immediately viable across several industries.
In addition, Guha said, “we envision that you could use multiple liquids and make much more complex emulsions.”
And besides being used in food, cosmetics, and drugs, the method could have other applications, such as in the oil and gas industry, where fluids such as the drilling “muds” sent down wells are also emulsions, Varanasi added.
© Copyright IBTimes 2024. All rights reserved.





















