Mars Crust Contributed To Its Atmosphere, NASA’s Curiosity Rover Finds

Thin and almost non-existent (compared to Earth) as the atmosphere surrounding Mars is, NASA’s Curiosity rover has found evidence that shows the red planet’s surface chemistry “contributed dynamically to the makeup of its atmosphere over time.” The findings were based on the analysis of gases xenon and krypton in Mars’ atmosphere.
An instrument suite called Sample Analysis Mars (SAM) onboard Curiosity studied the two gases, which “can be used as tracers to help scientists investigate the evolution and erosion of the Martian atmosphere,” according to a NASA statement. Previous knowledge about xenon and krypton in Mars’ atmosphere was based on the “analyses of Martian meteorites and measurements made by the Viking mission,” which was launched in the mid-70s.
Using a method called static mass spectroscopy — a good way to detect gases or isotopes that exist only in trace amounts — for the first time on the surface of another planet, scientists noticed that “some isotope ratios were a bit different than expected.” They realized that a process called neutron capture was responsible, in which the neutrons of one chemical element transferred to another within the surface material of Mars.
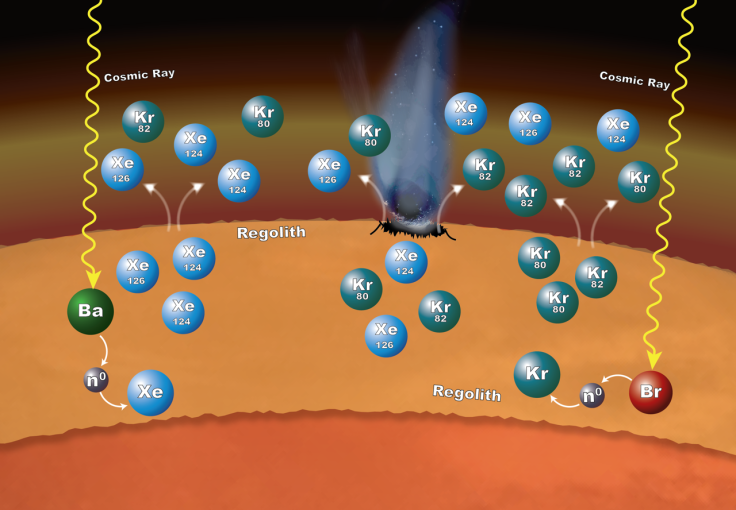
Specifically, the xenon-124 and -126 isotopes were seemingly formed with neutrons taken from barium, while krypton-80 and -82 isotopes appeared to have been formed through neutron capture involving bromine. These isotopes were probably released into the Martian atmosphere due to impacts on the surface and by gas escaping from the regolith.
“SAM’s measurements provide evidence of a really interesting process in which the rock and unconsolidated material at the planet’s surface have contributed to the xenon and krypton isotopic composition of the atmosphere in a dynamic way,” Pamela Conrad, lead author of the report and SAM’s deputy principal investigator at NASA’s Goddard Space Flight Center in Greenbelt, Maryland, said in the statement.
The experiments conducted by SAM to measure the xenon and krypton isotopes in Mars’ atmosphere were published in the journal Earth and Planetary Science Letters, under the title “In situ measurement of atmospheric krypton and xenon on Mars with Mars Science Laboratory.”
© Copyright IBTimes 2025. All rights reserved.





















