UK Distributes First AstraZeneca Vaccines: When Will The US Follow Suit?
KEY POINTS
- U.K. injects first-ever dose of AstraZeneca vaccine
- The government says it has access to more than 100 million doses
- The U.S. has delayed it over questions about the drug’s effectiveness
The United Kingdom on Monday said the first dose of the AstraZeneca vaccine against COVID-19 was a “pivotal moment” in its fight against the pandemic, though those in the U.S. may have to wait until April for the additional line of defense.
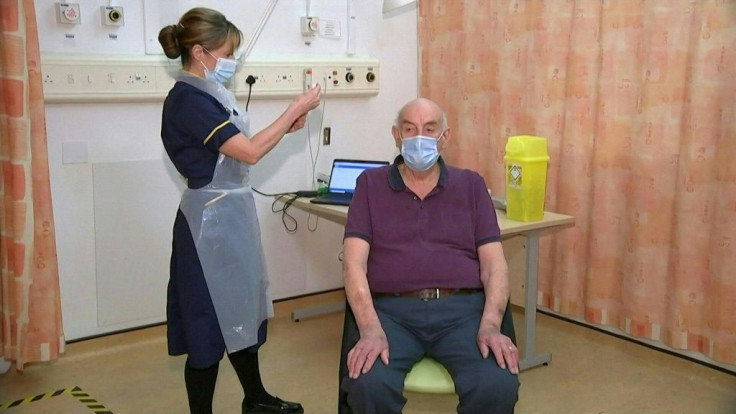
The British government announced its National Health Service was the first agency in the world to publicly distribute the AstraZeneca vaccine, which was developed with the support of the nation’s Oxford University.
“This is a pivotal moment in our fight against this awful virus and I hope it provides renewed hope to everybody that the end of this pandemic is in sight,” British Health Secretary Matt Hancock said in a statement.
On Monday, 82-year-old Brian Pinker became the first person to receive the Astra-Zeneca shot, part of a two-dose inoculation.
The British government said it has already secured access to more than 100 million doses of the Astra-Zeneca vaccine. Over half a million of those were ready for distribution as of Monday, and tens of million more are expected to be delivered in the coming weeks.
Those in the U.S., however, will have to wait. Last week, Moncef Slaoui, the head of the federal vaccine task force Operation Warp Speed, said the government was pushing back its approval for the AtraZeneca shot by at least two months, to April, given lingering concerns over the vaccine.
"The biggest question mark, frankly, is efficacy in the elderly population,” he was quoted by The Hill as saying. “That needs to be further documented just because there were not enough ... of the subjects recruited into trial.”
The AstraZeneca drug uses different methodology than the ones already in distribution in the U.S. The Moderna and Pfizer vaccines use RNA to trigger the body to make a protein that prompts an immune response, while AstraZeneca’s uses a weakened or inactivated viral strain to activate the body’s defense.
AstraZeneca’s vaccine may also be less effective than the others in use. The U.S. reluctance, however, comes amid questions about the nation’s own vaccine distribution campaign.
The U.S. government set a goal of having 20 million people inoculated with the first shot of a two-dose program by the end of December. The Centers for Disease Control and Prevention reported that the nation ended the month about 80% short of its goal.
Dr. Ashish Jha, the dean of Brown University’s School of Public Health, told the New York Times last week that the federal government has largely left it to overwhelmed state and local health officials to take up the task of vaccination.
“We’ve taken the people with the least amount of resources and capacity and asked them to do the hardest part of the vaccination -- which is actually getting the vaccines administered into people’s arms,” he said.
© Copyright IBTimes 2025. All rights reserved.




















