How To Use OraQuick - First Over-The-Counter HIV Test Approved By FDA
Americans will now be able to test themselves for HIV, the virus that causes AIDS, in the privacy of their own homes.
The U.S. Food and Drug Administration (FDA) on Tuesday announced it has approved OraQuick as the first over-the-counter in-home HIV test. It is a self-administered kit that detects the presence of HIV antibodies.
OraQuick is manufactured by OraSure Technologies, Inc. headquartered in Bethlehem, Pa. The retail price has not been announced.
Knowing your status is an important factor in the effort to prevent the spread of HIV, Dr. Karen Midthun, director of the FDA's Center for Biologics Evaluation and Research, said in a statement. The availability of a home-use HIV test kit provides another option for individuals to get tested so that they can seek medical care, if appropriate.
The Centers for Disease Control and Prevention estimates that 1.2 million people are living with the HIV infection in the United States. Moreover, one in five people don't know they are infected and there are approximately 50,000 new HIV infections every year.
The CDC said many of the new infections are passed on by people who are unaware of their status.
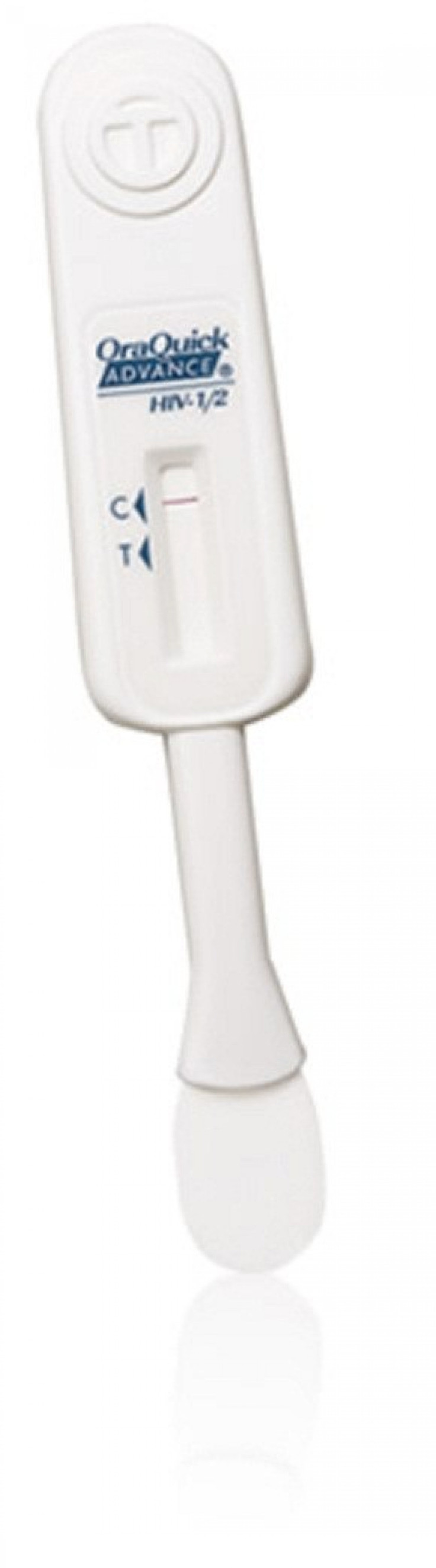
Here's how OraSure Technologies Inc.'s OraQuick HIV test kit works:
- The OraQuick In-Home HIV Test allows a person to collect an oral fluid sample by swabbing the upper and lower gums inside of his or her mouth.
- That sample is then placed in a developer vial and the results are obtained in 20 to 40 minutes.
- A positive result from the test doesn't definitely mean an individual is infected with HIV. Rather additional testing should be done by a medical professional to confirm the test result.
- Similarly, if the OraQuick test returns a negative result, it doesn't mean that a person isn't infected with the virus, especially when they may have been exposed within the past three months.
- The FDA said the test has the potential to find large numbers of previously undiagnosed HIV infections, especially if it is used by those unlikely to use the standard screening methods.
According to the FDA, clinical studies showed that when used properly OraQuick detected HIV in carriers 92 percent of the time. What that means is that there is one false negative result for every 12 infected people who use the kit.
The test kit was accurate 99 percent in ruling out HIV in non-carriers, according to information provided by the FDA. Therefore, the test would wrongly identify one patient as having HIV for every 5,000 HIV-negative people who uses it.
--
© Copyright IBTimes 2024. All rights reserved.












