Baidu.com, Goodyear Tire & Rubber, NXP Semiconductors, Telecom Italia, Elan Corp, Infosys Ltd, Banco Santander and Barclays Plc. are among the companies whose shares are moving in pre-market trading Tuesday.
Johnson & Johnson (NYSE: JNJ), the world's second-biggest health care company, is expected to report higher second-quarter profit as strong performance of new drugs help mitigate drags from generic competition and strings of recalls.
A new study offers a timeline of changes in spinal fluid, brain size, the appearance of brain plaques and other factors that precede the onset of Alzheimer's in people who are genetically predestined to develop the brain-wasting disease.
The Indian government has taken a major step toward its ambitious vision of achieving Universal Health Coverage (UHC) by initiating a $5.4-billion plan that would allow the government sector doctors to prescribe generic drugs to the patients free of cost.
A consumer group said Pfizer partly based its claims that Centrum products promote breast and colon health on the presence of vitamin D in the products, despite inconsistent or inconclusive evidence of vitamin D's protective role against breast and colon cancer.
From city hospitals to tiny rural clinics, India's public doctors will soon be able to prescribe free generic drugs to all comers, vastly expanding access to medicine in a country where public spending on health was just $4.50 per person last year.
GSK targeted the antidepressant Paxil to patients under age 18 when it was approved for adults only, and it pushed the drug Wellbutrin for uses it was not approved for, including weight loss and treatment of sexual dysfunction, according to an investigation led by the U.S. Justice Department.
Gamification - turning boring, unpleasant but necessary tasks into an online game - is a new way of thinking that is gaining momentum among drugmakers and health campaigners.
Among the companies whose shares are moving in pre-market trading Tuesday are: Ceragon Networks, Renren, Strategic Hotels & Resorts, Telefonica, EzChip Semiconductor, Banco Santander, Siemens, Pfizer and Goodyear Tire & Rubber.
Johnson & Johnson (NYSE: JNJ) has agreed to settle a probe into sales of its antipsychotic drug Risperdal and other medications for as much as $2.2 billion, Bloomberg reported Monday, citing two people familiar with the matter.
The emergence of drug-resistant or superbug strains of gonorrhoea is caused by unregulated access to and overuse of antibiotics, which helps fuel natural genetic mutations within the bacteria.
The companies whose shares are moving in pre-market trade Tuesday are: Popular Inc, North American Energy Partners Inc, United States Steel Corporation, Freeport-McMoRan Copper & Gold Inc, Pfizer Inc, Banco Santander S.A. and Facebook Inc.
A U.S. health advisory panel on Thursday issued a split vote on data for Pfizer Inc's drug to treat a rare neurodegenerative disease. The U.S. Food and Drug Administration panel voted 13 to 4 that the drug did not show that it met the main goal in a study in treating the fatal condition.
Portuguese investment bank Espirito Santo has downgraded Indian bio-technology major Biocon from neutral to sell based on several issues, including accounting practices which the bank says are objectionable.
Experimental drugs that show a big effect early in development for treating serious or life-threatening diseases would get a faster and cheaper path to U.S. approval, under a proposal likely to become law this year. U.S. drug regulators would be able to label such treatments breakthrough therapies, and work with companies to speed up clinical trials, for example by testing the drugs for a shorter time or enrolling fewer patients.
The companies whose shares are moving in pre-market trade Wednesday are: Protalix BioTherapeutics, Charming Shoppes, Tripadvisor, Genworth Financial, Wynn Resorts, UBS AG, Herbalife, CBS Corp, Patni Computer Systems and Chesapeake Energy Corp.
Futures on major US stock indices point to a lower opening Tuesday ahead of economic data, which include ISM manufacturing index.
The first-quarter earnings season has so far generated big waves of positive surprises, which fueled the upward momentum in the stock market. However, investors should take these earnings beats with a grain of salt.
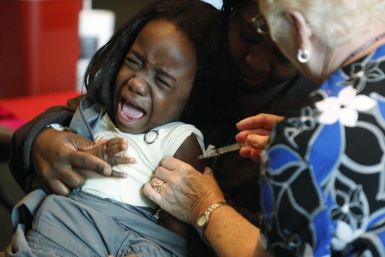
When Odei Antwi-Agyei had the chance of introducing vaccines to prevent Ghana's children dying of diarrhea, or vaccines to stop them dying of pneumonia, he did what no African immunization chief has done before. He said he'd do both at the same time.
Swiss food group Nestle SA may sell 10 to 15 percent of the $11.9 billion baby food business it is buying from Pfizer Inc to address antitrust concerns, Bloomberg News reported Monday.
Swiss food group Nestle is to buy drugmaker Pfizer's baby food business for $11.85 billion, beating out French rival Danone in the battle for dominance of fast-growing emerging markets.
Nestle SA, the world's largest food group, said Monday that it has agreed to acquire children's food maker Pfizer Nutrition for $11.85 billion.