Recall Alert: Over 50,000 COVID-19 Rapid Test Kits Sold Without Approval Recalled
KEY POINTS
- The rapid tests may yield "inaccurate test results": FDA
- Customers are being advised not to use the kits
- Rapid tests are a "fast and convenient" COVID-19 test option
A company has recalled its antigen rapid test kits as they were reportedly distributed without the necessary approval. More than 50,000 units are affected.
The products were distributed without "premarket clearance or approval," the U.S. Food and Drug Administration (FDA) announced on its website. This may lead to "inaccurate test results due to lack of performance evaluation by the FDA."
Universal Meditech voluntarily recalled the product after "becoming aware of the violative distribution notified by the FDA," the FDA said.
Customers have been advised to not use the products.
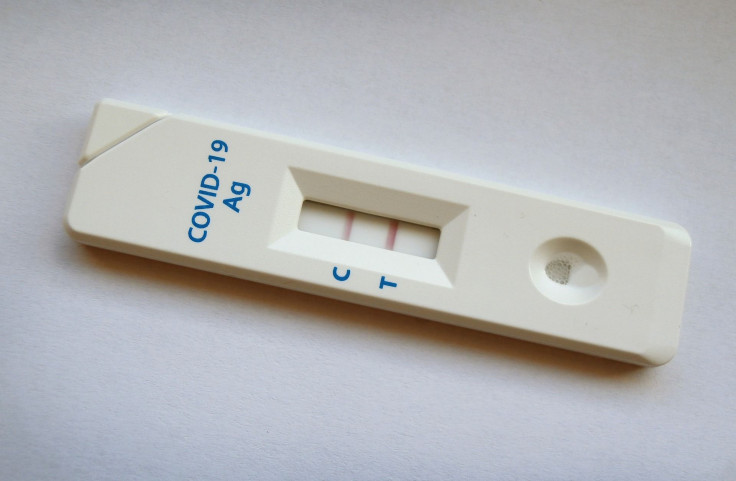
The recall affects Universal Meditech's Skippack Medical Lab SARS-CoV-2 antigen rapid test kits. They were cassette-model COVID-19 tests that came in three different packages: a purple and white box with the brand name "Skippack Medical Lab," a green and white box with the brand name "DiagnosUS" and a white box without a brand label.
Sample photos of the packaging are available on the FDA's website. So far, there have been no reports of injuries related to the recall.
The recall affected about 56,300 units. The recalled products were made from October to December 2021 and distributed in California and Texas.
Customers with questions about the recall can call +1(702)871-9888 and can contact the distributor to return the products.
COVID-19 rapid tests from another company were recalled last year for similar reasons. More than 209,000 units of SML Distribution's Skippack Medical Lab SARS-CoV-2 antigen rapid test kits were affected at the time. They were distributed in several states, including Florida, Georgia and New York.
Antigen tests, also called rapid tests or at-home tests, detect the protein (antigens) from the SARS-CoV-2 virus, the FDA explained. Generally, the agency advises repeat testing after a negative test result — even if one is symptom-free — to "increase the accuracy." This is to lessen the risk of getting a false negative test result and to avoid inadvertently spreading the virus to others.
"COVID-19 diagnostic testing remains a cornerstone of our nation's fight against COVID-19," the agency said. "At-home COVID-19 antigen tests, while not perfect, provide a fast and convenient COVID-19 testing option."
© Copyright IBTimes 2025. All rights reserved.





















