Biotech News: New Drug To Treat Elephant Man Disease In Children Wins FDA Approval
KEY POINTS
- The phase 2 trial was conducted by the National Cancer Institute, Cancer Therapy Evaluation Program
- Koselugo was developed by Merck and AstraZeneca for treatment of inoperable plexiform neurofibromas
- The condition is caused by the mutation of the NF1 gene
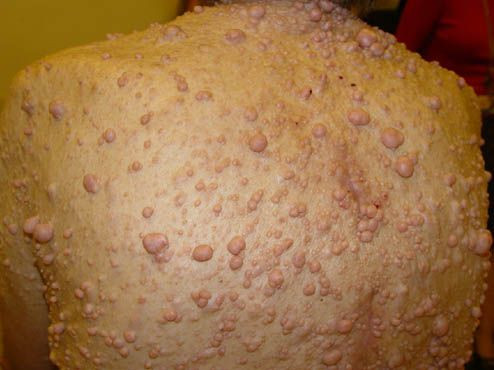
The Food and Drug Administration greenlighted the first drug developed to treat children suffering from elephant man disease, a rare and painful nerve condition in which tumors form within the protective sheaths surrounding nerves.
AstraZeneca Plc (AZN) and Merck & Co. (MRK) said Monday they received approval for Koselugo, a kinase inhibitor, to treat neurofibromatosis type 1 in cases where it is impossible to remove plexiform neurofibromas with surgery. The condition causes pain, motor dysfunction, breathing problems, bowel and bladder issues, and disfigurement.
“For the first time, patients and families impacted by this incurable genetic condition have an approved medicine to treat the resulting plexiform neurofibromas,” Dave Fredrickson, executive vice president for AstraZeneca’s oncology business unit, said in a press release.
“This approval has the potential to change how symptomatic, inoperable NF1 plexiform neurofibromas are treated and provides new hope to these patients,” added Dr. Roy Baynes, senior vice president, head of global clinical development and chief medical officer for Merck Research Laboratories.
The phase 2 drug trial was conducted by the National Cancer Institute, Cancer Therapy Evaluation Program.
The drug companies reported a 66% response rate – 33 of 50 pediatric patients with the condition –with 20% of patients in complete or partial tumor reduction within six months. Patients received the drug twice daily and the effects lasted a year or longer in 82% of patients who showed response, the FDA said.
Koselugo, which was given an orphan drug designation, caused serious adverse side effects, including cardiomyopathy, ocular toxicity, gastrointestinal toxicity, skin toxicity, increased creatinine phosphokinase (CPK), increased vitamin E levels and risk of bleeding, and fetal harm, with 82% of test subjects reporting vomiting and 80% suffering from rashes. Abdominal pain (76%) and diarrhea (70%) also were reported.
Neurofibromatisos type 1 is a genetic disease that affects 3,000 to 4,000 individuals and can reduce life expectancy by 15 years. Some 30% to 50% of those born with the condition develop plexiform neurofibromas.
There was no immediate word on the treatment’s cost.
© Copyright IBTimes 2025. All rights reserved.






















