The Tightest Molecular Knot Ever Created Is 10,000 Times Thinner Than A Human Hair
Trying to knot tiny molecules together is a task that is exactly as difficult as it sounds. However, manipulating molecules into ever tighter knots is a goal that many researchers eagerly pursue, and not just for the daunting challenge it provides.
Scientists believe that making different types of molecular knots can be used as a method to probe how knotting affects strength and elasticity of materials. This, in turn, can lead to the creation of polymer strands that can be used to build stronger and more flexible materials.
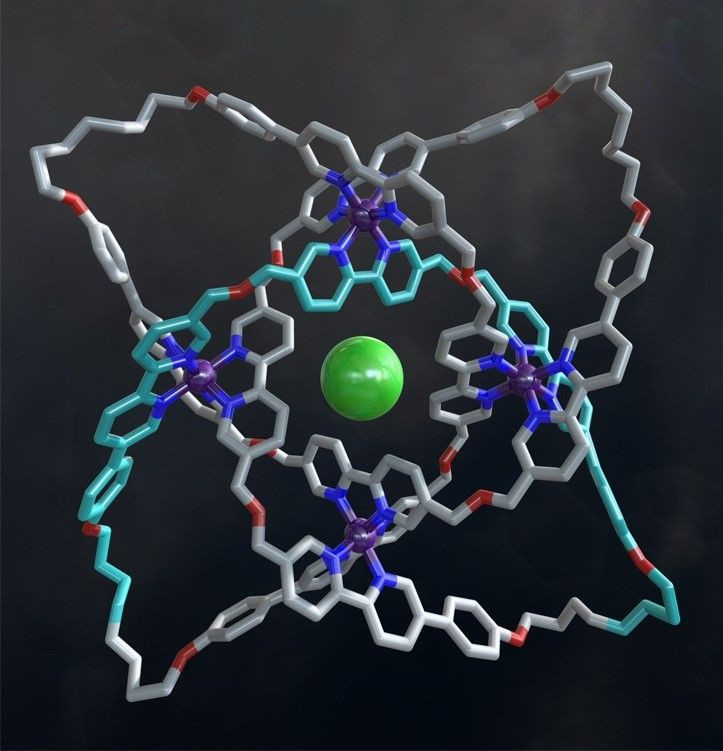
A team of researchers from the University of Manchester has now created a record-breaking knot. It contains eight crossings in a 192-atom closed loop, and is roughly 10,000 times thinner than a human hair — the most tightly knotted physical structure ever created.
“Tying knots is a similar process to weaving, so the techniques being developed to tie knots in molecules should also be applicable to the weaving of molecular strands,” David Leigh from the University of Manchester, one of the authors of the a study detailing the achievement, said in a statement. “For example, bullet-proof vests and body armor are made of Kevlar, a plastic that consists of rigid molecular rods aligned in a parallel structure — however, interweaving polymer strands have the potential to create much tougher, lighter and more flexible materials in the same way that weaving threads does in our everyday world.”
The researchers created the knot using a technique called “self-assembly,” wherein molecular strands are woven around metal ions, forming crossing points in the right places — in this case, eight places. The ends of the strands were then fused together by a chemical catalyst to close the loop.
“The strands that we’re knotting are so small that you can’t grab the ends and mechanically tie them like you would a shoelace,” Leigh, who was part of the first team that created a synthetic pentafoil knot in 2011, told New Scientist. “Unlike tying your shoelaces, where you do them one at a time, with self-assembly we can tie many billions of molecular knots at the same time.”
The end product contained, among other elements, chlorine, hydrogen and nitrogen atoms, and its structure was confirmed using X-ray crystallography.
© Copyright IBTimes 2025. All rights reserved.





















