According to a 2024 report by the Office of the National Coordinator for Health Information Technology, 96% of non-federal acute care hospitals in the United States have adopted certified EHRs.
Far more than another tech product, WorldCare.ai positions itself as a worldwide movement, a bold stand against broken systems, bureaucratic gatekeeping, and AI gimmickry.
Elisa C. Alvarado, M.D, notes how advances in diagnostics and medicine now offer more targeted relief, and lifestyle adjustments can further support recovery.
When one thinks of solutions to global poverty, eye care rarely makes the list. But it should, and Seva Foundation has the research to prove it.
Behind every misuse or abuse is a story of trauma, mismanaged pain, misguided treatments, and people caught in a system that seems to forget their humanity.
Sleep apnea is not just a health issue but rather a business problem, costing billions in lost productivity, increased healthcare claims, absenteeism, and on-the-job accidents. WhisperSom℠, co-founded by Michael G. Nathans, is providing a potential solution to tackle sleep apnea, a breathing disorder that carries cumulative health risks, some even fatal.
Cost founded the Now You See Me Foundation, a nonprofit dedicated to supporting Texas-based athletes, particularly cyclists and runners, who suffer catastrophic injuries while training.
Nowhere is this more critical than in oncology, where every day and every decision can change the course of a patient's life. Anna Forsythe, founder of Oncoscope-AI, an AI-powered oncology intelligence platform, has spent her career at the intersection of science, economics, and clinical practice.
At its heart, 340B is about getting medicine—and the healthcare services needed to ensure their safe and effective use—to people who otherwise might go without it.
Founded in 2013 and headquartered in Warwickshire, YouNeek Productions began as a one-man passion project and has since evolved into a full-service creative agency working with global clients across sectors.
Melchor's philosophy is rooted in his journey with Parkinson's. From a single tremor in 2020 to a diagnosis two years later, this battle was as challenging as it was illuminating.
Affinity Triangle Therapy's services are tailored for individuals, couples, and families. Operating on a telehealth model, the practice provides flexible solutions for those balancing busy schedules, geographic limitations, or mobility challenges within a tiered pricing model, making care more accessible.
In 2025, the cost of employer-sponsored health coverage is expected to rise by 9%, surpassing $16,000 per employee. This would put organizations under extreme financial pressure.
Helix BioPharma is a clinical-stage biopharmaceutical company aiming to turn scientific breakthroughs into real-world solutions for some of the most difficult-to-treat cancers.
Dr. DePue attributes the growing interest in therapies like ketamine to a societal shift toward holistic, patient-empowered care.
By leveraging AI to analyze the millions of pixels in standard medical scans, Floy extracts valuable health insights that would otherwise remain undetected.
Live Love Life is a tax-exempt nonprofit that provides vital healthcare, education, and support to individuals battling HIV, hepatitis C, and other acquired diseases.
Employee burnout has been common even before the pandemic, but after the world was shut down by COVID-19, the wellbeing of employees has become a central point of discussion in the corporate world. If you're one of those employers who recognize the importance of worker wellbeing, these platforms and apps may be what you need to transform your workplace in 2025.
Unlike traditional treatments like chemotherapy and radiation, which often harm healthy cells, Immunocine's approach is precise, attacking only the cancerous cells while preserving the body's overall health.
Stay safe with the iHealth Flu A&B/COVID-19 3-in-1 Rapid Test
Learn about the differences and testing for flu A and B and COVID-19 and how to make holiday travels and gatherings safer.
With the right stylish essentials, you can transform rooms into inviting retreats that exude everyday luxury
In 2019, Americans faced $3.14 billion in out-of-pocket costs for breast cancer treatment, making it the most costly cancer treatment category.
Switching to a bidet, like the LUXE Bidet® NEO Plus series, can improve comfort, especially for those with sensitive skin or certain medical conditions, while promoting a cleaner and greener lifestyle
Drawing upon decades of behavioral research, ikHerstel developed a digital platform that uses real-world data and transparent artificial intelligence to provide adaptive individualized recovery plans.
Researchers found significant differences in health outcomes across generations, pointing to a concerning trend known as the "generational health drift."
Pacific Coast Mental Health's treatment plans incorporate more well-established therapies such as Cognitive Behavioral Therapy (CBT), Dialectical Behavior Therapy (DBT), and psychodynamic therapy.
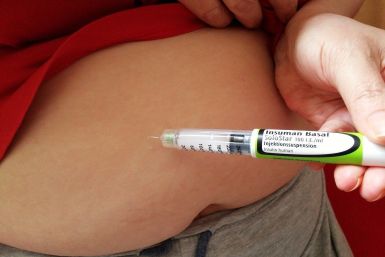
The company claims the Zepbound doses cost 50% less than competing drugs
Weight-loss drugs produced and distributed by Eli Lilly show promising results in cutting long-term diabetes risk.
According to the FDA's guidance document on when to submit a 510(k) for changes to an existing device, manufacturers must submit a new 510(k) if there are significant changes to the device's design, materials, or intended use.