COVID-19 Treatment: FDA Won't Use Blood From Gay And Bisexual Recovered Coronavirus Patients
KEY POINTS
- COVID-19 patients who recover have antibodies in their blood
- They can donate blood for clinical trials testing a coronavirus cure
- The FDA bars gay and bisexual men from donating blood
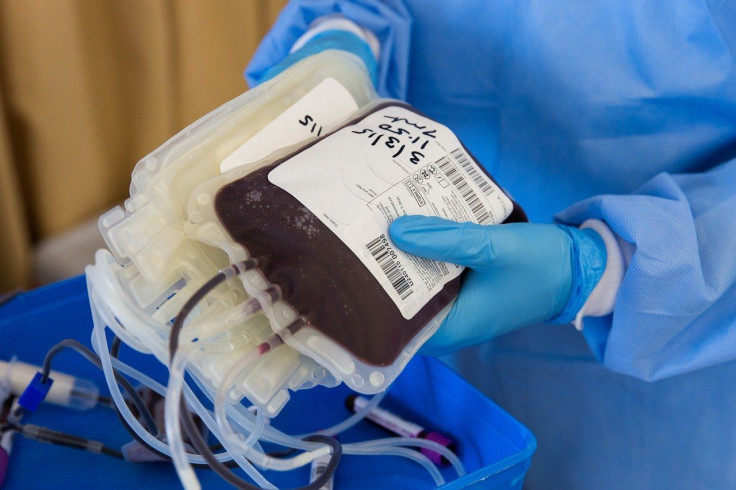
COVID-19 patients who have recovered develop antibodies that may be helpful in finding a treatment for coronavirus. However, the Food and Drug Administration (FDA) is barring some patients from donating their blood in plasma clinical trials if they are gay or bisexual.
The FDA has existing guidelines that disallow men who have sexual relations with other men to donate blood as a safety measure against HIV transmission. It used to be a lifetime ban, but the FDA revised its policy as a 12-month restriction in 2014.
A spokesperson from the FDA confirmed that the policy remains unchanged.
"We are actively considering the situation as the outbreak progresses," the spokesperson said.
According to the American Red Cross, thousands of their blood drive from last month were canceled due to the coronavirus quarantine. Thus, there is a shortage of blood plasma in clinical trials or convalescent plasma trials, which could potentially develop a cure for coronavirus. Doctors are testing and injecting the blood plasma with antibodies to those who are still sick with COVID-19.
NYBCe, 1st to collect blood plasma donations from recovered COVID-19 patients to treat severe cases. "If this treatment proves to be effective, we are prepared to quickly scale our process and serve hospitals nationwide" Beth Shaz, MD. Read More Here--> https://t.co/kehA7hpseD pic.twitter.com/3pFy6JdVt2
— Comprehensive Cell Solutions,New York Blood Center (@CcsNybc) March 25, 2020
In response to the FDA, Democratic senators wrote the agency a letter asking a change in the "antiquated policy" on blood donations.
"This antiquated policy is not based on current science, stigmatizes the LGBTQIA+ community, and undermines crucial efforts to increase the nation’s blood supply as the United States grapples with the coronavirus crisis," the senators, led by House Oversight and Reform Committee Chairwoman Carolyn Maloney, D-Ny., and Rep. Alexandria Ocasio-Cortez, D-Ny., wrote.
The FDA’s spokesperson confirmed that they received the letter from the senators.
GLAAD President and CEO Sarah Kate Ellis are also asking the FDA to lift the ban in a petition that has earned over 18,000 signatures so far.
"The FDA cannot let an outdated and discriminatory ban on blood donations from gay and bi men get in the way of potentially life-saving treatment for the country's painful current health crisis," Ellis said. "Continuing to enforce this antiquated policy is dangerous, irresponsible, and flies in the face of recommendations from medical experts."
Yesterday, @US_FDA confirmed that its guidelines that prevent gay and bisexual men, as well as others in the LGBTQ community, from donating blood during the COVID-19 crisis also applies to plasma donations.https://t.co/CEUD5AZRS4
— GLAAD (@glaad) April 1, 2020
© Copyright IBTimes 2025. All rights reserved.





















