US FDA Approves Over-the-counter Sale Of Overdose Reversal Drug Narcan
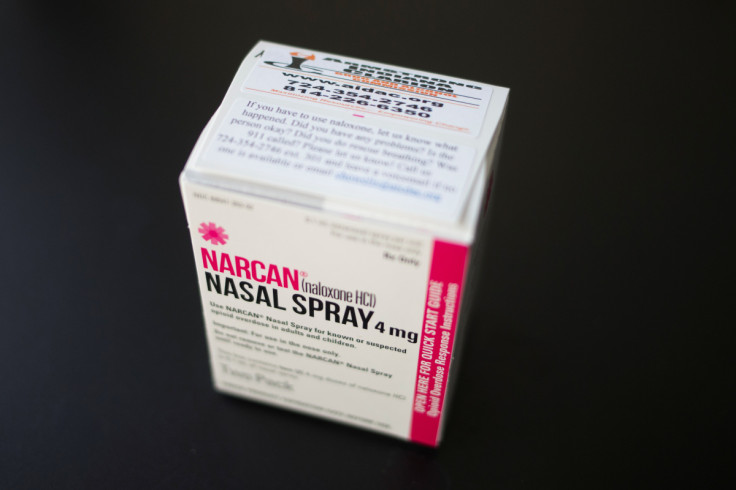
The U.S. Food and Drug Administration on Wednesday approved over-the-counter (OTC) sales of Emergent BioSolutions Inc's Narcan, allowing for easier availability of the life-saving medication used to reverse opioid overdoses.
The formal decision makes Narcan the first naloxone-based drug available without a prescription and will help align the federal government's stance with states that already offer the nasal spray without prescription at pharmacies.
Emergent said it will make Narcan available on U.S. store shelves and online retailers by late summer.
"I think it's a big win...in terms of getting this medication into places where previously people could not purchase it. The question now remains about the cost," said Noa Krawczyk, assistant professor at the NYU Grossman School of Medicine.
The contract drugmaker declined to share details on the price of its OTC version of Narcan. Krawczyk said approval was "half the battle" in terms of making the drug accessible.
Naloxone rapidly reverses or blocks the effects of opioids, restoring normal respiration, especially when given within minutes of the first signs of an overdose.
U.S. drug-related overdose deaths rose about 15% year-over-year to more than 100,000 in 2021, according to government estimates.
The FDA approval followed a unanimous recommendation from its independent panel of advisers backing OTC use of Narcan, while suggesting changes to the drug's packaging.
Shares of Maryland-based Emergent rose as much as 20.2% to $10.70 in early trading before paring gains.
The Biden administration has been pushing for action against drug addiction and overdose, especially due to restrictions and underfunding surrounding key tools such as naloxone and syringe service programs.
The approval puts Emergent ahead in the OTC product race. While Benchmark analyst Robert Wasserman, ahead of the FDA green light, noted that Narcan sales had been declining, he added, "I do think there'll be a big demand for the product and I don't think it's going to be too price sensitive right away either."
Emergent reported a 14% fall in 2022 for its nasal naloxone products, compared with a 40% increase in 2021. In late 2021, FDA approved the first generic version of Narcan, sold by Israeli drugmaker Teva Pharmaceuticals.
Non-profit Harm Reduction Therapeutics' application for its OTC naloxone nasal spray is currently under FDA review with a decision expected in July 2023.

© Copyright Thomson Reuters 2024. All rights reserved.





















