Warp Speed Director Has Questions About Vaccine Side Effects
KEY POINTS
- Moderna says its drug is 'well-tolerated'
- The vaccine is no peace of cake, one health official said
- Vaccines could be widely available by the middle of next year
Some of the short-term side effects of a COVID-19 vaccine are similar to other injected treatments but the long-term reactions aren’t yet clear, the head of the White House vaccine task force said Tuesday.
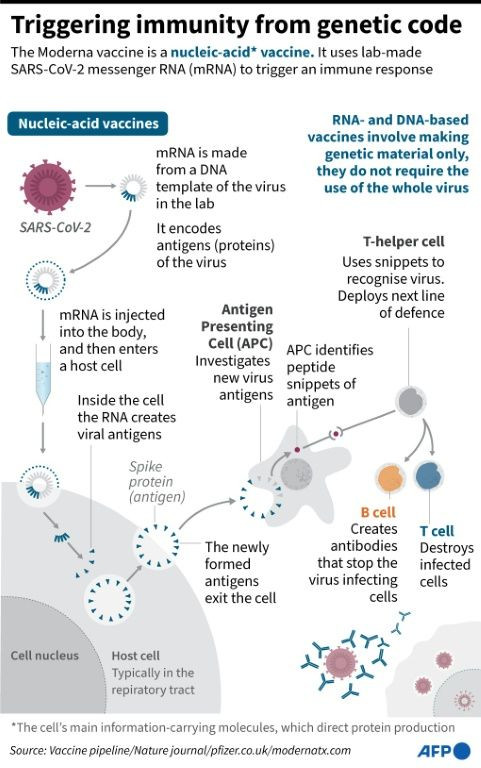
Pharmaceutical company Moderna is the latest in a growing list of those seeking emergency-use authorization for its genetic-based two-dose cocktail that would immunize against COVID-19. The company on Monday said its vaccine has a 94.1% efficacy rate.
Its drug, based on messenger RNA, “continues to be well-tolerated,” the company said in a statement.
In a live-panel discussion Tuesday with the Washington Post, Dr. Moncef Slaoui, the head of the White House Operation Warp Speed task force, said some of the short-term side effects – pain at the injection side, chills, a fever and muscle aches – are well known.
“The longer, more important kind of adverse events such as some autoimmune disease or others have not been reported in a different way between the placebo group and the vaccine group in these two trials, which is very reassuring,” he said. “I always make sure we say that [while] we know the short term and I’m going to call it mid-term effects of the vaccine is now well understood, the very long-term safety is not yet understood by definition.”
Vaccines based on messenger RNA, or mRNA, are novel and represent a breakthrough in immunology. At issue with these particular cocktails is they require more than one dose to prove effective.
Dr. Sandra Fryhofer of the American Medical Association advised the Centers for Disease Control and Prevention that the medical community was concerned some people may not follow through with the second dose because of the adverse side effects of the first one.
“We really need to make patients aware that this is not going to be a walk in the park,” Fryhofer said.
Those who participated in clinical trials for Moderna and Pfizer/BioNTech told CNBC in September the short-term side effects could be intense, from fatigue to a high fever, but they usually went away within a day. It was better, they said, than contracting COVID-19.
News of vaccine developments could be encouraging people to let their guard down. Dr. Anthony Fauci, the top U.S. infectious disease expert, said most people who want a vaccine can get one as early as mid-2021.
“If I recommend one thing [for the holiday season] it’s that we diminish, to the extent possible, travel — and keep gatherings indoor to the immediate family unit,” he said in an interview Monday with Facebook CEO Mark Zuckerberg.
© Copyright IBTimes 2025. All rights reserved.





















