Coronavirus Treatment: FDA Issues Emergency Authorization For Use Of Convalescent Plasma
KEY POINTS
- Convalescent plasma has shown preliminary high efficacy in treating coronavirus patients
- Although not yet clinically tested, FDA issued an emergency use authorization to use it for treatment
- The supply is limited because it can only come from patients who have recovered from COVID-19
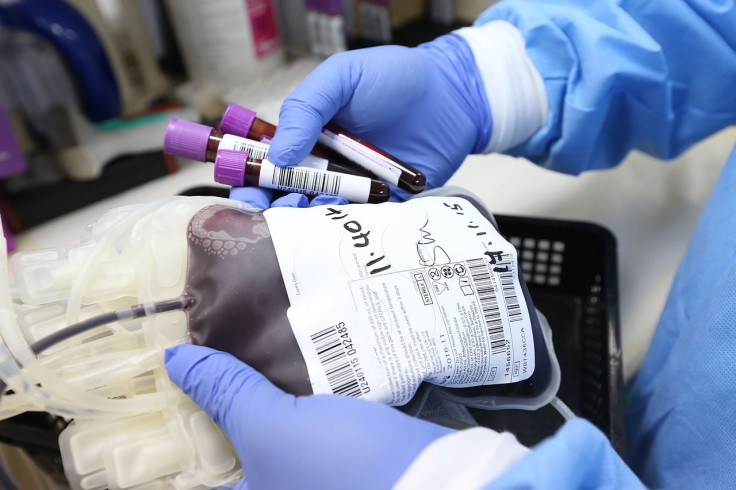
After much debate on whether to officially use convalescent plasma, the U.S. Food and Drug Administration (FDA) issued an emergency use authorization (EUA) for the use of convalescent plasma in treating COVID-19 patients.
The FDA announced its issuance of an emergency use authorization so doctors could start treating coronavirus patients with convalescent plasma, the yellowish liquid part of the blood that carries water, enzymes and salt. The primary role of plasma is to deliver hormones, proteins and nutrients to the sections of the body that need it.
Emergency use authorization is one of the powers given to the FDA under the Federal Food, Drug and Cosmetic Act, which gives authority to the agency to allow the utilization of unapproved products for use in an emergency to treat, diagnose, or prevent life-threatening diseases or conditions when there are no other available or adequate alternatives. This authority is further strengthened by various congressional amendments, such as the Pandemic and All-Hazards Preparedness Reauthorization Act of 2013.
Convalescent plasma traditionally is the go-to treatment in cases where there are no other alternatives. It is collected from patients who have already recovered from a particular disease and are presumed to have created antibodies against such ailments. These antibodies help the patient fight off the infection caused by the illness.
Some doctors say there are strong indicators that survivor plasma may help in fighting COVID-19 infection, although there are no solid scientific proofs of such assertions.
In March, the FDA established a pathway for researchers to study convalescent plasma and its impact on coronavirus patients. Records indicate that over 70,000 COVID-19 patients have already been treated with convalescent plasma.
However, the supply of convalescent plasma is limited since it must come only from donors who already recovered from COVID-19 infection. Furthermore, while there are promising indicators from convalescent plasma studies, there are currently no randomized clinical trial data that can establish with certainty the efficacy of the therapy.
The FDA, in its announcement allowing the use of the therapy Sunday, said that the known and potential benefits of convalescent plasma outweigh the possible risks. The agency also revealed that thousands of patients have already received convalescent plasma as treatment, CNN reported.
In a separate statement, President Donald Trump expressed happiness with the FDA's decision. "Today I am pleased to make a truly historic announcement in our battle against the China virus that will save countless lives. Today's action will dramatically increase access to this treatment," Trump said during a briefing at the White House.
Recently, the president hurled accusations at some health officials, whom he said are "playing politics" when it comes to a convalescent plasma EUA. When asked then about the agency's non-issuance of the EUA, the president said the reason was political.
On Sunday, CNN reported that an unnamed official close to the White House Coronavirus Task Force revealed that the agency reviewed additional data to help it arrive at its EUA decision. This official did not see, nor review, the data in question, but said that the FDA is under no obligation to ask for consultations from anyone outside the agency as regards it EUA decision.
© Copyright IBTimes 2025. All rights reserved.





















