FDA Approves BTG's Voraxaze Drug for Cancer Toxicity
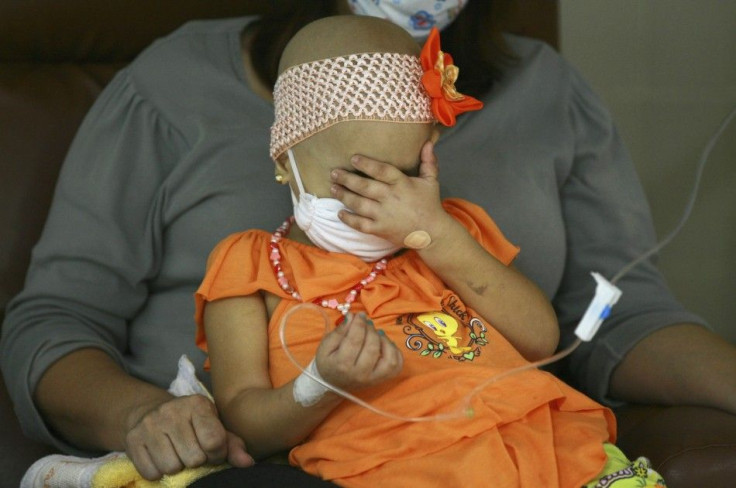
WASHINGTON (Reuters) - U.S. health regulators gave the nod on Tuesday to a drug from British specialty drugmaker BTG Plc that helps cancer patients get rid of toxic levels of a chemotherapy treatment.
The drug, called Voraxaze, helps eliminate methotrexate in patients whose kidney function has been compromised by treatment with high doses of the chemotherapy agent. Methotrexate is normally eliminated from the body by the kidneys, but prolonged high doses of the drug used to treat cancer can result in kidney failure.
BTG's injectable treatment can quickly break down the chemotherapy medicine and allow the body to expel it.
The Food and Drug Administration granted Voraxaze orphan drug status, meant for rare diseases or conditions that affect a very small portion of the population. As incentive for companies to develop such drugs, the orphan designation comes with seven years of marketing exclusivity before a rival medicine could be approved.
Methotrexate is used to treat breast, bone and lung cancer as well as leukemia. In much lower doses, it is commonly used to treat rheumatoid arthritis and other autoimmune diseases.
Prolonged exposure to the chemotherapy treatment can cause kidney and liver damage, skin rash and severe mouth sores, damage to the lining of the intestines, and death because of low blood counts, said Richard Pazdur, the head of the FDA's cancer drugs division.
Voraxaze is an important new treatment option for cancer patients aimed at preventing these toxicities associated with sustained high levels of methotrexate, Pazdur said in a statement.
In a clinical trial of 22 patients, Voraxaze eliminated 95 percent of methotrexate from their blood. For 10 of the patients, the methotrexate fell to a low level within 15 minutes and stayed that way for eight days, the FDA said.
Common side effects included low blood pressure, headaches, nausea and vomiting.
(Additional reporting by Bill Berkrot in New York; Editing by Bernard Orr)
© Copyright Thomson Reuters {{Year}}. All rights reserved.






















