FDA Revises Blood Donation Deferral Guidelines Amid COVID-19; Lifts Limits For LGBTQ Donors
Movement Aims To Reduce Stigma on HIV/AIDS, Gay, Bisexual Blood Donors; Response To Prevent Nationwide Blood Supply Shortage
KEY POINTS
- FDA revises 12-month ban period for man having sex with men donors
- Apply also to donors with tattoo, piercings
- Reduced deferral to 3 months
- Response to calls to prevent U.S. blood shortage amid COVID-19
- Hope to remove stigma on donors
- Estok, 37, COVID-19 survivor rejected from donation because of gender
- Estok, prime candidate for convalescent plasma
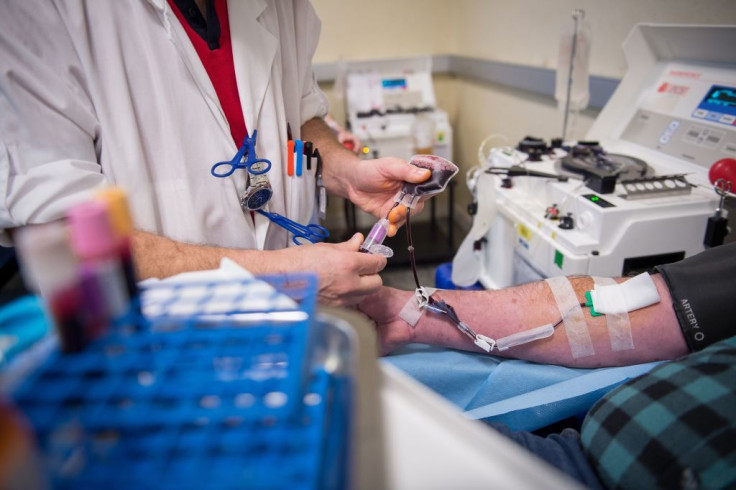
To prevent a blood supply shortage in the U.S. amid the coronavirus pandemic, the U.S. Food and Drug Administration (FDA) made revisions on the guidelines regarding blood donor deferrals on April 2.
Due to calls from the Surgeon General for healthy Americans to donate blood to provide a consistent supply, the FDA revised the 12-month ban period for gay and bisexual men to practice sexual abstinence before donating blood to a much shorter time of three months, ABC News reported.
The recent revision would apply effectively to gay men and bisexual men in monogamous relationships, to gay men and bisexual men practicing safe sex, to gay men and bisexual men who are HIV-negative, women who have had sex with men who also engaged in sexual activities with other men, and those individuals who had tattoos or piercings.
And while according to the FDA that the decision to revise the deferral period was based on “recently completed studies and epidemiologic data”, doctors all over the U.S. said that any deferral period during this time is not only a stigmatization of the LGBTQ+ community, but also, a limiting factor to the country’s critical blood supply.
The #COVID19 pandemic has impacted blood donation in the US due to several factors, including cancelled blood drives. The nation’s blood supply relies on healthy donors to give blood. Donation is safe & easy & can be done without risk to donors. https://t.co/NreJog7Bc8 https://t.co/BUjdEMpxVZ
— U.S. FDA (@US_FDA) March 19, 2020
The original policy was a lifetime ban from donating blood created amid the HIV/AIDS crisis in the 1980s and because of the limited tests and medical technology at the time, the FDA imposed the lifetime ban in 1983 on male donors who had sexual activities with other men since 1977.
The guidelines were first revised in 2015 when the agency reduced the lifetime ban to a year-long or 12-month ban period where the blood donor should abstain from having any sexual relations with a man during the said time interval.
In her interview with Good Morning America, Dr. Monica Hahn of U.C. San Francisco Health, who had been specializing in HIV Prevention and Management, said that due to modern technology in HIV testing, there is no scientific reason to have this deferral anymore and it is only the stigma that keeps the policy in place.
“These bans were made because it was a frightening time where we just didn't have the testing technology to adequately screen our blood supply,” Hahn said.
“It would take several months after someone acquired HIV for it to show up on a test because your body needs time to make antibodies in reaction to the HIV virus,” she added. “But, if you fast forward to today with our newer tests, the window period is really reduced to just about 10 days, meaning that we can really accurately detect the presence of HIV in patients about 10 days after HIV transmission occurs. The onus is on our screening system and I really believe that people are being discriminated against.”
Recently, a viral post on Facebook circulated about Lukus Estok, 37, a COVID-19 survivor who was a prime candidate of a New York City hospital as a potential blood donor with high plasma coronavirus antibody count but was then rejected from donating due to his sexual orientation.
“I was subsequently put through two separate (but identical) rounds of donor screening questions,” Estok wrote. “I answered the questions honestly. The New York Blood Center scheduled an appointment for me to donate my plasma.”
“At that moment, the facial expression, even with a mask on in the look of the eyes of the person I was talking to, completely changed. And without any hesitation, the response back to me was, ‘well, you will not be donating today,’” Estok said.
See posts, photos and more on Facebook.
However, despite the guidelines being revised to have looser restrictions over the years, they are still guideline and not official mandates and therefore, it is still up to blood centers to uphold the stricter guidelines or to impose the revised ones.
The New York Blood Center, where Estok was expected to give his plasma, told the show that implementing FDA’s revised guidelines is not an immediate process, however, they said that they are working as quick as they can and the implementations would be finished in the middle of May.
“We’ve been in contact with the donor [Lukus] to discuss how we can further improve our donor engagement processes,” the New York Blood Center spokesperson said. “We would love to work with him to advocate for further change, and we would welcome him to come back and be at the front of the line when we begin accepting newly eligible donors in the coming weeks."
Dr. Hahn said that the U.S. is already behind countries such as Italy and Africa who have employed “individual risk assessment” when it came to the probability of HIV transmission through blood and blood products; in fact, according to the U.S. National Library of Medicine National Institutes For Health, Italy had “moved from a permanent deferral for men having sex with men to an individual risk assessment of sexual behaviors”.
American Medical Association Dr. Patricia Harris agreed to Dr. Hahn, saying that “risk-based approach should be data-driven” such as giving potential donors questionnaires asking about recent sexual history, recent injection of non-prescription drugs and even past or recent exposure to people with infectious diseases.
“The AMA has been a long-term advocate of using a risk-based approach rather than stigmatizing one group of people. So we believe there should not even be the three-month deferral, but that we should use a risk-based approach,” Harris told “GMA.”
In an open letter Dr. Hahn penned to the FDA with her UCSF colleague Dr. Deborah Cohan, she urged them for “scientifically-driven standards that uphold the utmost safety of the blood supply and simultaneously promote equity and reverse historical discrimination in blood donation.”
Dear @US_FDA,
— GLAAD (@glaad) April 1, 2020
Americans need blood and plasma, but millions can't give.
End the blood ban: https://t.co/8tBWWsBZYX pic.twitter.com/DvaNRlxUcz
Crises like #COVID19 don’t discriminate. Our blood donation policies shouldn’t either.
— Human Rights Campaign (@HRC) April 22, 2020
Add your name to @HRC’s letter demanding the FDA change its blood donation policy related to #LGBTQ men.https://t.co/AibauOmk0w
.@sarahkateellis, president & CEO of @glaad, discusses the FDA ban on blood donations from gay and bisexual men. https://t.co/6GtIoekOe1
— ABC News Live (@ABCNewsLive) March 28, 2020
"It's outdated...I think that what this does is just reinforce the notion that there's something wrong with being gay or being bisexual." pic.twitter.com/JDPyhfeiYB
However, America’s Blood Centers, the organization that operates North America’s largest network of independent, community-based blood centers such as the New York Blood Center, to be careful when using the term “immediate effect” used by the FDA when it comes to implementing the revisions and the policies as it might cause confusion for the public.
“The word ‘immediate’ means that blood centers may begin the process of implementing the changes, all of which happens in a regulated and controlled environment,” America’s Blood Centers CEO Kate Fry told “GMA.” For a blood center, this means significant updates to an FDA-reviewed donor history questionnaire, SOPs/procedures, computer systems, and trainings. Blood centers also will need to validate their procedures, computer systems, and trainings.”
An FDA spokesperson said that the agency is committed to finding an alternative to the deferral placed on men having sex with men by studying scientific data that would support an effective individual risk assessment-based blood questionnaire and added that they working to start on a “pilot study to enroll 2,000 men who have sex with men and who would be willing to donate blood”.
© Copyright IBTimes 2025. All rights reserved.





















